 CSF Glucose - an overview | ScienceDirect Topics
CSF Glucose - an overview | ScienceDirect TopicsAFP IssuesAFP By Colecciones Tópicas AFP CME QuizAFP Community BlogSubscribe to AFPCerebrospinal Fluid AnalysisDEAN A. SEEHUSEN, M.D., MARK M. REEVES, M.D., and DEMITRI A. FOMIN, M.D., Tripler Army Medical Center, Honolulu, HawaiiAm Fam Physician. 2003 Sep 15;68(6):1103-1109. Lumbar puncture is often performed in primary care. Properly interpreted tests can make the cerebrospinal fluid (CSF) a key tool in the diagnosis of a variety of diseases. The proper evaluation of the CSF depends on knowing which tests should be ordered, normal ranges for the patient's age, and the limitations of the test. The level of protein, opening pressure and the relationship of CSF-a-sound glucose vary with age. Xanthochromia is most often caused by the presence of blood, but several other conditions must be considered. The presence of blood can be a reliable predictor of subarachnoid bleeding but it takes several hours to develop. The three tube method, commonly used to rule out a central nervous system bleeding after a "traumatic tap", is not completely reliable. Red blood cells in the CSF caused by a traumatic tap or subarachnoid hemorrhage artificially increase the count of white blood cells and the level of protein, thus confusing the diagnosis. Diagnostic uncertainty can be reduced using accepted corrective formulas. The white blood cell differential can be deceptive early in the course of meningitis, as more than 10 percent of cases with bacterial infection will have an initial lymph predominance and viral meningitis can initially be dominated by neutrophils. Culture is the gold standard to determine the cause organism in meningitis. However, the polymerase chain reaction is much faster and more sensitive in some circumstances. Latex aglutination, with high sensitivity but low specificity, may have a role in managing partially treated meningitis. To test herptic, cryptococcal or tubercular infection, special staining techniques or collection methods may be required. Primary care doctors usually perform lumbar puncture, because cerebrospinal fluid (CSF) is an invaluable diagnostic window for the central nervous system (CNS). Tests commonly performed in CSF include protein and glucose levels, cell and differential counts, microscopic examination and culture. Additional tests can also be performed such as opening pressure, supernatant color, latex aglutination and polymerase chain reaction. Knowing what tests to order and interpret them allows doctors to use CSF as a key diagnostic tool in a variety of diseases. Opening Pressure To measure the opening pressure of CSF, the patient must be in the side position of the legs and neck in a neutral position. The meniscus will fluctuate between 2 and 5 mm with the patient's pulse and between 4 and 10 mm with breaths. The patient should be advised not to stop, because the effort can increase the opening pressure, and warned that he would not hyperventilate, because the hyperventilator will lower the opening pressure. Normal opening pressure varies from 10 to 100 mm H2O in young children, 60 to 200 mm H2O after eight years, and up to 250 mm H2O in obese patients. Intracranial hypotension is defined as an opening pressure of less than 60 mm H2O. This finding is rare except in patients with a history of trauma that causes a CSF leak, or when the patient has had a previous lumbar puncture. Opening pressures above 250 mm H2O are diagnosis of intracranial hypertension. High intracranial pressure is present in many pathological states, including meningitis, intracranial bleeding and tumors. Idiopathic intracranial hypertension is a condition that is most commonly seen in obese women during their child-raising years. When a high opening pressure is discovered, the CSF must be removed slowly and the pressure monitored during the procedure. No additional CSF should be removed once the pressure reaches 50 percent of the opening pressure. Supernatant ColorNormal CSF is crystalline. However, as few as 200 white blood cells (WBCs) per mm3 or 400 red blood cells (RBCs) per mm3 will cause the CSF to appear turbide. Xanthochromia is a yellow, orange or pink discoloration of the CSF, most of the times caused by the lysis of RBCs resulting in the decomposition of hemoglobin to oxihemoglobin, methemoglobin and bilirubin. The discoloration begins after the RBCs have been in spinal fluid for about two hours, and remains for two to four weeks. Xanthochromia is present in more than 90 percent of patients within 12 hours of subarachnoid bleeding and in patients with serum bilirubin levels between 10 to 15 mg per dL (171 to 256.5 μmol per L). CSF protein levels of at least 150 mg per dL (1.5 g per L)—as seen in many infectious and inflammatory conditions, or as a result of a traumatic tap containing more than 100,000 RBCs per mm3—it will also result in xanthochromia. The newborn CSF is often xanthochromic due to the frequent elevation of bilirubin and protein levels in this age group. list the CSF colors associated with several conditions. Blending of blood Blending of blood Reference information , and .Cell countNormal CSF can contain up to 5 WBCs per mm3 in adults and 20 WBCs per mm3 in newborns. 87% of patients with bacterial meningitis will have a WBC count of more than 1,000 mm3 while 99% will have more than 100 per mm3. Having less than 100 WBCs per mm3 is more common in patients with viral meningitis. WBC's high counts can also occur after a seizure, intracerebral hemorrhage, malignity, and a variety of inflammatory conditions. list the common findings of FCR in various types of meningitis. Typical brain fluids at various types of meningitisTestBacterialViralFungalTubercularOpening Pressure Normally Normally Variable Variable White blood cell count ≥1,000 per mm3 Opening pressure Normally normal Variable Variable White blood cell count ≥1,000 per mm3Voending Variable cellular dominance* Predominence of lymphocytes Predominence of protein lymphocytesMild to elevation marked Normal to elevated ElevatedCSF-to-serum glucose ratioNormal to marked decreaseUsually normalLowCSF = cerebrospinal fluid; PMNs = polymorphocytes.*—Linfocytosis present 10 percent of the time.†— PMNs can predominate early in the course. Reference Information ,, and .Typic Cerebrospinal Fluid Findings in Various Types of MeningitisTestBacterialViralFungalTubercularOpening PressureElevated Normally Normally Variable Variable White cell count ≥1,000 per mm3 Opening pressure Normally Variable Variable Variable cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular cellular Predominence of lymphocytes Predominence of protein lymphocytesMild to elevation marked Normal to elevated ElevatedCSF-to-serum glucose ratioNormal to marked decreaseUsually normalLowCSF = cerebrospinal fluid; PMNs = polymorphocytes.*—Linfocytosis present 10 percent of the time.†— PMNs can predominate early in the course. The reference information, and .Peripheral blood in the CSF after a "traumatic tap" will result in an artificial increase in the WBCs by a WBC for every 500 to 1,000 RBCs in the CSF. This correction factor is accurate as long as the WBC peripheral count is not extremely high or low. A traumatic tap is produced in about 20 percent of lumbar scores. Common practice is to measure cell counts in three consecutive CSF tubes. If the number of RBCs is relatively constant, then the blood is supposed to be caused by intracranial bleeding. A fall count is attributed to a traumatic tap. However, the three-tube method is not always reliable. Xanthochromia is a more reliable predictor of bleeding. If a traumatic tap occurs within 12 hours of suspicious subarachnoid bleeding, it is reasonable to repeat the lumbar puncture one interspatial until trying to get a clear CSF. Cellular Difference The WBC count seen in the normal adult CSF is composed of approximately 70 percent lymphocytes and 30 percent monocytes. Occasionally, a lone eosinophilic or polymorphocyte (PMN) will be seen in the normal CSF. Several NPMs in the CSF of a neonatal patient is not unusual. Most patients with Guillain-Barré syndrome will have 10 or less monocytes per mm3 and a minority of patients will have between 11 and 50 monocytes per mm3. Up to 50 monocytes per mm3 are seen in about 25 percent of patients with multiple sclerosis. Cell differential alone cannot differentiate between bacterial and non-bacterial meningitis. Lymphocytosis is observed in viral, fungal, and tuberculosis infections of CNS, although a predominance of NPMs may be present in the early stages of these infections. CSF in bacterial meningitis is typically dominated by the presence of PMNs. However, more than 10 percent of bacterial meningitis cases will show a lymph predominance, especially early in the clinical course and when there are less than 1,000 WBCs per mm3 (). Eosinophilic meningitis is defined as more than 10 eosinophils by mm3 or a total CSF cell count composed of more than 10% eosinophils. Parasitic infection must be suspected in this situation. Other possible causes may include viral, fungal or rickettsial meningitis; having ventriculoperitoneal eruptions with or without a co-existing infection; malignancy; and adverse drug reactions. Microscopic examination The prophylactic stain is positive in 60 to 80 percent of the nontreated cases of bacterial meningitis and in 40 to 60 percent of the partially treated cases. Sensitivity according to the causative organism ranges from 90 percent in pneumoccal or staphylococcal meningitis to less than 50 percent in Listeria meningitis. Hifae may occasionally be seen in Candida or other cases of fungal meningitis. Several factors influence the sensitivity of the Gram stain. The laboratory techniques used to concentrate and stain CSF can greatly influence reliability. Cytocentrifugation increases the ability to detect bacteria. Increased number of colony forming units (CFU) per CSF mm3 increase the probability of a positive result. The content will be positive in 25% of cases if there are less than 1,000 UF per mm3 and 75% of cases if there are more than 100,000 UF per mm3 present. Finally, the experience of laboratory staff is very important. Up to 10 percent of Gram's initial stains are misread. Acid-fast staining should be done if tuberculosis is clinically suspected. Only 37 percent of the initial smears will be positive for acid-fast bacilli. This result can be increased to 87% if four smears are made. Sensitivity can also be increased by examining the CSF sediment. Other stains should be made if indicated by the situation. Cryptococcus can be identified up to 50 percent of the time in Indian ink preparation. Current water control should always be done to ensure that India's ink is not contaminated. Toxoplasmosis can be diagnosed with Wright or Giemsa stain. A simple CSF wet preparation under a cover slide can produce positive results in a variety of protozoan and helmintic infections. Protein LevelCSF concentration is one of the most sensitive indicators of pathology within the CNS. Newborn patients have up to 150 mg per dL (1.5 g per L) of protein. The adult range from 18 to 58 mg per dL (0.18 to 0.58 g per L) is reached between six and 12 months of age. The doctor should know what the normal reference range is for your laboratory, because the measurement is somewhat dependent on the technique. High CSF protein is observed in infections, intracranial bleeding, multiple sclerosis, Guillain Barré syndrome, malignities, some endocrine abnormalities, a certain use of medicines and a variety of inflammatory conditions (). Protein concentration is falsely elevated by the presence of RBCs in a traumatic tap situation. This can be corrected by subtracting 1 mg per dL (0.01 g per L) of protein per 1,000 RBCs per mm3. [Evidence level B: observational study] This correction is only accurate if the same tube is used for protein and cell counts. Average and range of Cerebrospinal (0.13-2.0) Brain fluid in nervous system diseases. 2d ed. Philadelphia: Saunders, 1992. (0.13-228,0) Brain spinal fluid in nervous system diseases. 2d ed. Philadelphia: Saunders, 1992. Low levels of CSF proteins can occur in conditions such as repeated lumbar puncture or chronic leakage, in which CSF is lost at a higher than normal rate. The low levels of CSF proteins are also observed in some children from six months to two years, in acute water poisoning, and in a minority of patients with idiopathic intracranial hypertension. CSF protein levels do not fall into hypoproteinemia. Glucose level A true normal range cannot be given for CSF glucose. As a general rule, CSF glucose is approximately two thirds of the serum glucose measured during the previous two or four hours in a normal adult. This relationship decreases with the increase in serum glucose levels. CSF glucose levels generally do not exceed 300 mg per dL (16.7 mmol per L) regardless of serum levels. Glucose in the CSF of neonates varies much more than in adults, and the CSF-a-sero ratio is generally higher than in adults. CNS infections can cause low-glucose levels of SCR, although glucose levels are generally normal in viral infections (). Normal levels of glucose do not rule out infection, because up to 50 percent of patients who have bacterial meningitis will have normal levels of CSF glucose. Chemistry meningitis, inflammatory conditions, subarachnoid bleeding and hypoglycemia also cause hypoglycemia (low glucose level in the RSF). High blood glucose levels are the only cause of a high level of CSF glucose. There is no pathological process that will elevate CSF glucose levels. CultureCultures made in 5 percent of sheep's blood grab and enriched chocolate agar remain gold standards to diagnose bacterial meningitis. Antibiotic treatment prior to lumbar puncture may decrease the sensitivity of culture, especially when given intravenously or intramuscularly. enterovirus, the main cause of viral meningitis, can be recovered in 40 to 80 percent of cases. The culture for the simple herpes virus is 80 to 90 percent sensitive but can take five to seven days to be positive. The results of viral cultures rarely change the initial management of meningitis. Mycobacterium tuberculosis is best cultivated using multiple large volume samples of CSF. It is recommended at least 15 mL and preferably 40 to 50 mL of CSF. Culture is positive 56 percent of the time in the first sample, and improved up to 83 percent of the time if four separate samples are cultivated. These cultures usually take up to six weeks to identify positively. Fungal cultures are positive in more than 95 percent of cases of Cryptococcus neoformans and in 66 percent of cases of candidal meningitis. Other fungi are less likely to be positive culture. Similar to tuberculosis meningitis, cultural performance in fungal meningitis can be increased by obtaining large volumes of CSF through repeated lumbar scores. Latex Aglutination The latex aglutination (LA) allows the rapid detection of bacterial antigens in the CSF. Sensitivity varies a lot between bacteria. LA for Haemophilus influenzae has a sensitivity of 60 to 100 percent, but it is much lower for other bacteria. The specificity for LA is very low. However, LA can be useful in cases of partially treated meningitis where cultures cannot produce an organism. Because false positives lead to unnecessary treatment, LA is not routinely used today. Some experts suggest using LA in cases of suspicious bacterial meningitis if the initial prophylactic stain and bacterial culture are negative after 48 hours. Polymerase chain reaction The polymerase chain reaction (PCR) has been a major advance in the diagnosis of meningitis. PCR has high sensitivity and specificity for many CNS infections, is fast, and can be done with small CSF volumes. Although the tests are expensive, there is a cost-saving potential decreasing overall diagnostic tests and intervention. PCR has been particularly useful in the diagnosis of viral meningitis. CSF PCR has a sensitivity of 95 to 100 percent, and a sensitivity of 100 percent for the herpes simple virus type 1, Epstein-Barr virus, and enterovirus. PCR is faster and more sensitive than culture for meningitis enterovirus. When PCR is positive for enterovirus, it allows the previous hospital discharge and less intervention. [Evidence level B: retrospective chart review]PCR is the most sensitive means of diagnosing CNS CMV infections, and it has been suggested that the RCP will replace brain biopsy as the gold standard for herpes encephalitis. PCR has a sensitivity of 54 to 100 percent and a specificity of 94 to 100 percent for tuberculosis meningitis, and could replace acid-fast bacillus and culture as a test of choice. The RCP is sensitive to acute neurosyphilis but not for more chronic forms. PCR is also being studied as a diagnostic tool for bacterial meningitis and other CNS infections. Read the full article. Read the full article. Already a member/subscriber? Purchase Access: Already a member or subscriber? Best value! Get full access from $140 Access This Issue$39.95 Already a member or subscriber? The best value! Get full access This issueThe AuthorsDEAN A. SEEHUSEN, M.D., is a faculty development student at the Family Practice Department of the Madigan Army Medical Center, Tacoma, Wash. Previously, he was a doctor of the staff of the Department of Family Practice and Emergency Medical Services at Tripler Army Medical Center, Honolulu. He obtained his degree in medicine from the University of Iowa College of Medicine, Iowa City, and completed a family practice residence in Tripler Army Medical Center....MARK M. REEVES, M.D., is director of the family practice residency program at Tripler Army Medical Center. He obtained his medical degree from the University of Uniformed Services of Health Sciences, Bethesda, Md., and completed a residence in family practice in Dwight D. Eisenhower Army Medical Center, Augusta, Ga.DEMITRI A. FOMIN, M.D., is a staff neurologist from the Department of Medicine, neurology service, in Tripler Army Medical Center. He obtained his medical degree from the University of Uniformed Health Science Services and completed a residency in neurology at the Walter Reed Army Medical Center, Washington, D.C.A. correspondence with Dean A. Seehusen, M.D., 5803 152nd Ave. Ct. E, Sumner, WA 98390 (e-mail:). The prints are not available from the authors. The views and statements contained in this document are the private views of the authors and should not be interpreted as official or as a reflection of the views of the United States Army Medical Corps or the United States Army in general. The authors indicate that they have no conflicts of interest. Sources of funding: not reported. REFERENCES1. Lyons MK, Meyer FB. Physiology of brainspinal fluid and increased intracranial pressure management. May Clin Proc. 1990;65:684–707....2. Fishman RA. Brain spinal fluid in nervous system diseases. 2d ed. Philadelphia: Saunders, 1992.3. Khurana RK. Intracranial hypotension. Semin Neurol. 1996;161:5–10.4. Conly JM, Ronald AR. Brain fluid as a diagnostic body fluid. I'm J Med. 1983;751B:102-8.5. Dougherty JM, Roth RM. Brain spinal fluid. Emerg Med Clin North Am. 1986;4:281–97.6. Ahmed A, Hickey SM, Ehrett S, Trujillo M, Brito F, Goto C, et al. Values of the brain fluid in the term neonate. Pediatr Infect Dis J. 1996;15:298–303.7. Morgenlander JC. Lumbar punch and CSF exam. Postgraduate Med. 1994;958:125–31.8 Edlow JA, Caplan LR. Avoid difficulties in diagnosing subarachnoid bleeding. N Engl J Med. 2000;342:29–36.9. Treseler CB, Sugar AM. Fungal meningitis. Infect Dis Clin Norte Am. 1990;4:789–808.10. Arevalo CE, Barnes PF, Duda M, Leedom JM. Brainspinal fluid cells count and pharmacies in bacterial meningitis. South Med J. 1989;82:1122-7.11. Weller PF, Liu LX. Eosinophilic meningitis. Semin Neurol. 1993;13:161-8.12. Kaplan SL. Clinical presentations, diagnosis and prognostic factors of bacterial meningitis. Infect Dis Clin Norte Am. 1999;13:579–94.13. Pruitt AA. Nervous system infections. Neurol Clin. 1998;16:419-47.14. Niu MT, Duma RJ. Protozoa and helminth meningitis. Infect Dis Clin Norte Am. 1990;4:809–41.15. Leonard JM, Des Prez RM. Tuberculosis meningitis. Infect Dis Clin Norte Am. 1990;4:769–87.16. Davis LE. Fungal infections of the central nervous system. Neurol Clin. 1999;17:761–81.17. Wubbel L, McCracken GH Jr. Management of bacterial meningitis: 1998. 1998;193:78–84.18. Lee SJ, Kurtz JB. Laboratory diagnosis of common viral infections of the central nervous system using a single multiple PCR detection test. J Clin Microbiol. 1999;37:1352-5.19. Greenlee JE. Approach to the diagnosis of meningitis. Evaluation of the brain fluid. Infect Dis Clin Norte Am. 1990;4:583–98.20. Zunt JR, Marra CM. Brainspinal fluid tests for diagnosis of central nervous system infection. Neurol Clin. 1999;17:675–89.21. Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A. Diagnosis of complications of the central nervous system in HIV-infected patients: analysis of cerebrospinal fluid due to the reaction of the polymerase chain. AIDS. 1997;11:1–17.22. Tanel RE, Kao SY, Niemiec TM, Loeffelholz MJ, Holland DT, Shoaf LA, et al. Prospective comparison of the detection of culture vs genome for the diagnosis of enteroviral meningitis in childhood. Arch Pediatr Adolesc Med. 1996;150:919–24.23. Ramers C, Billman G, Hartin M, Ho S, Sawyer MH. Impact of a reaction test of the brain spinal fluid polymerase chain diagnosis in patient management. JAMA. 2000;283:2680-5.24. Jeffery KJ, Lee SJ, I'll take you. Mayon-White RT, Bangham CR. Diagnosis of viral infections of the central nervous system: clinical interpretation of RCP results. Lancet. 1997;349:313-7.25. Garcia-Monco JC. Central nervous system tuberculosis. Neurol Clin. 1999;17:737-59. Copyright © 2003 by the American Academy of Family Physicians. This content is owned by AAFP. A person who sees it online can make an impression of the material and can use that impression only for his personal and non-commercial reference. This material cannot be downloaded, copied, printed, stored, transmitted or reproduced in any medium, either now known or later invented, except as authorized in writing by AAFP. Contact for copyright questions and/or permission requests. You want to use this article somewhere else? More in AFPEditor's collectionsLearn more in PubmedMOST RECENT ISSUEMar 1, 2021Mar 1, 2021Access to the latest issue of American Family PhysicianEmail Alerts Don't miss a single problem. Subscribe to the AFP free content table. Browse this articleContinue reading Previous: Previous: Next: / / / / Analysis of brain fluids Copyright © 2020 American Academy of Family Physicians. All rights reserved.
Web: maycliniclabs.com E-mail: mcl@mayo.edu Telephone: 800-533-1710 International: +1 855-379-3115 Values are valid only on the day of printing. Test ID: Glucose GLSF, Spinal Fluid To investigate possible infections of the central nervous system Clinical information The brainspinal fluid (CSF) is secreted by croid plexies, around the brain vessels, and along the walls of the ventricles of the brain, filling the ventricles and cisternae and bathing the spinal cord. The CSF is reabsorbed in the blood through the arachnoid villi. The CSF billing is fast, exchanging about 4 times a day. CSF glucose levels can be reduced due to the consumption by microorganisms, the transport of glucose with deficiencies or the increase of glucolysis. High levels of CSF glucose are consistent with hyperglycemia. Reference values The concentration of glucose in spinal fluid should be approximately 60% of the plasma/suero concentration and should be compared to glucose in plasma/suero measured simultaneously for appropriate clinical interpretation. For the unit SI Reference values, see Interpretation The levels of brainspinal fluid glucose (CSF) may be decreased in any central nervous system infection, although the levels are normally normal in viral meningitis, low in bacterial meningitis, and may be normal or low in fungal meningitis. CSF glucose levels are usually about 60% of blood glucose levels. Cautions Handle specimens in containers stopped to avoid contamination and evaporation. Brainspinal fluid specimens should be processed without delay; they may contain cellular components, as well as organisms, which reduce glucose concentration over time. Clinical reference Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. Edited by CA Burtis, ER Ashwood, DE Bruns, St. Louis, MO, Elsevier Saunders, 2012© 1995–2021 Mayo Foundation for Medical Education and Research. All rights reserved.

Summary of literature search for reference ranges of CSF glucose,... | Download Table
Bacterial meningitis will have a high opening pressure + WBC's present in the CSF (predominately neu… | Meningitis, Medical laboratory science, Bacterial meningitis
Child Neurology: Differential diagnosis of a low CSF glucose in children and young adults | Neurology
Typical Cerebrospinal Fluid Findings in Various Types of Meningitis | Download Table
Diagnostic challenges with acellular bacterial meningitis
Cerebrospinal Fluid Glucose and Lactate: Age-Specific Reference Values and Implications for Clinical Practice
Evaluation of csf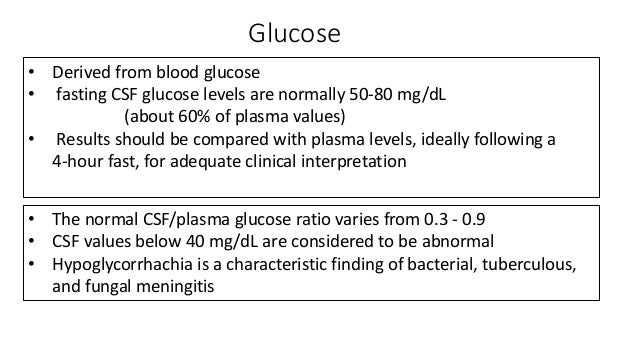
Csf composition and significance by Dr. Ashok KUmar J
Cerebrospinal fluid cytological and biochemical characteristics in the presence of CNS neoplasia
404 Not Found | Nursing school, Nurse, Glucose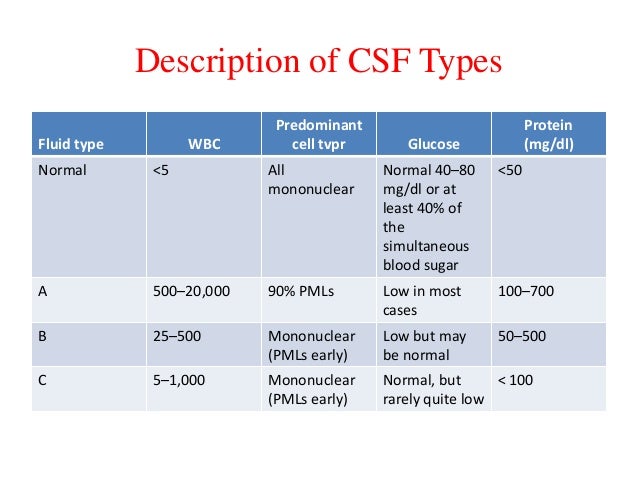
Csf seminar
BODY FLUIDS: Cerebrospinal Fluid - ppt video online download
Cerebrospinal Fluid Supernatant Colors and Associated Conditions or Causes | Download Table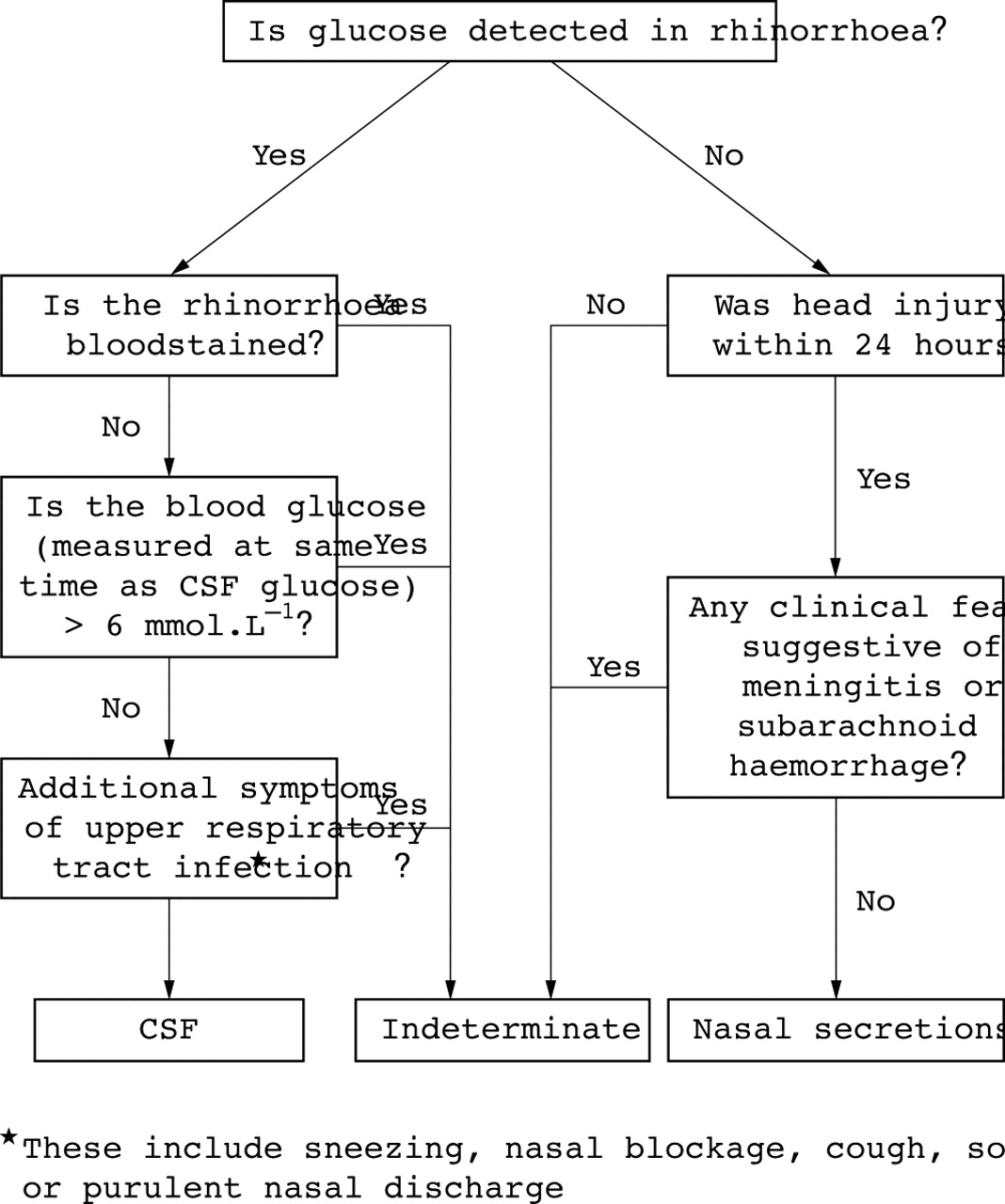
New insights into the glucose oxidase stick test for cerebrospinal fluid rhinorrhoea | Emergency Medicine Journal
Cerebrospinal fluid analysis acute bacterial versus viral meningitis
Causes of albuminocytological dissociation and the impact of age-adjusted cerebrospinal fluid protein reference intervals: a retrospective chart review of 2627 samples collected at tertiary care centre | BMJ Open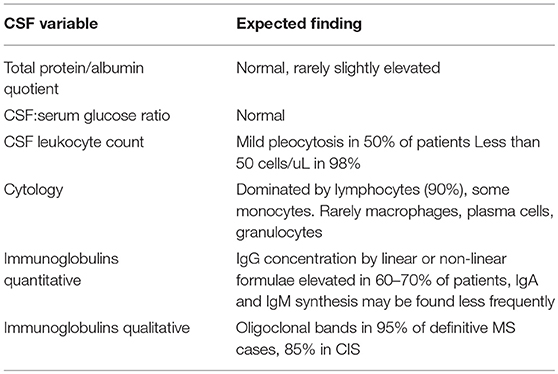
Frontiers | The Cerebrospinal Fluid in Multiple Sclerosis | Immunology
Laboratorial diagnosis of lymphocytic meningitis
Cerebrospinal Fluid Glucose and Lactate: Age-Specific Reference Values and Implications for Clinical Practice
Cerebrospinal Fluid Supernatant Colors and Associated Conditions or Causes | Download Table
CSF ANALYSIS. - ppt download
Pleocytosis is not fully responsible for low CSF glucose in meningitis
PLOS ONE: G-CSF Protects Human Brain Vascular Endothelial Cells Injury Induced by High Glucose, Free Fatty Acids and Hypoxia through MAPK and Akt Signaling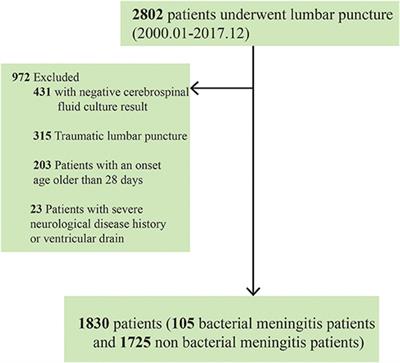
Frontiers | Comparing Single vs. Combined Cerebrospinal Fluid Parameters for Diagnosing Full-Term Neonatal Bacterial Meningitis | Neurology
PLEOCYTOSIS
Csf analysis presentation
Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer's disease detected by dynamic glucose-enhanced MRI | Science Advances
Laboratorial diagnosis of lymphocytic meningitis
Glucose Level - an overview | ScienceDirect Topics
Diagnostic challenges of central nervous system infection: extensive multiplex panels versus stepwise guided approach - Clinical Microbiology and Infection![PDF] Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. | Semantic Scholar PDF] Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0ff234eca141e30a3adaa7d7e74b3379fc78dadd/3-Figure1-1.png)
PDF] Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. | Semantic Scholar
Type-2-diabetes Alters CSF but not Plasma Metabolomic and AD Risk Profiles in Vervet Monkeys | bioRxiv
Relationship between the levels of CSF glucose/HbA1c and the severity... | Download Scientific Diagram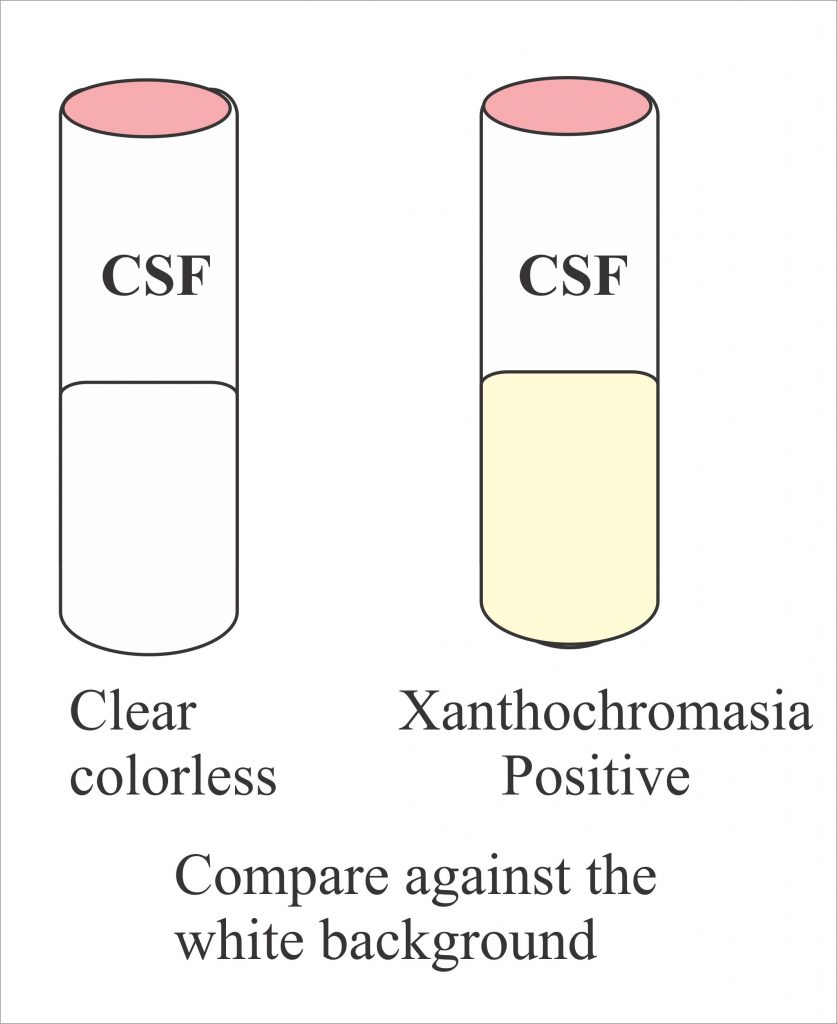
Cerebrospinal Fluid Analysis – Part 2 – (CSF), Complete Examination, Normal / Abnormal, – Labpedia.net
Cerebrospinal Fluid Reference Values for Young Infants Undergoing Lumbar Puncture | American Academy of Pediatrics
Laboratorial diagnosis of lymphocytic meningitis
Repeat lumbar puncture in adults with bacterial meningitis - ScienceDirect
Diagnostic Approach to Health Care- and Device-Associated Central Nervous System Infections. - Abstract - Europe PMC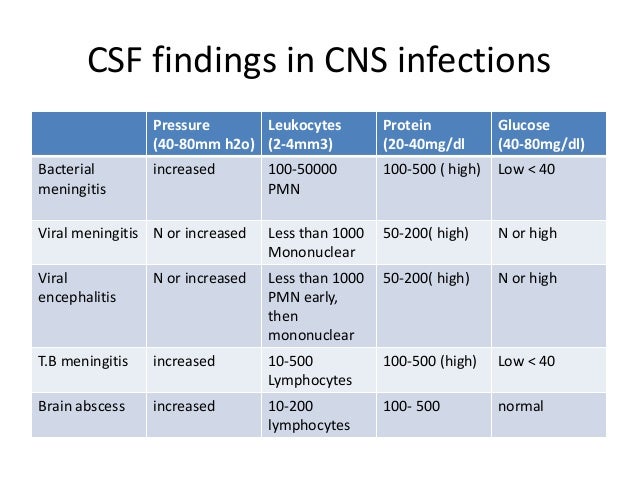
Csf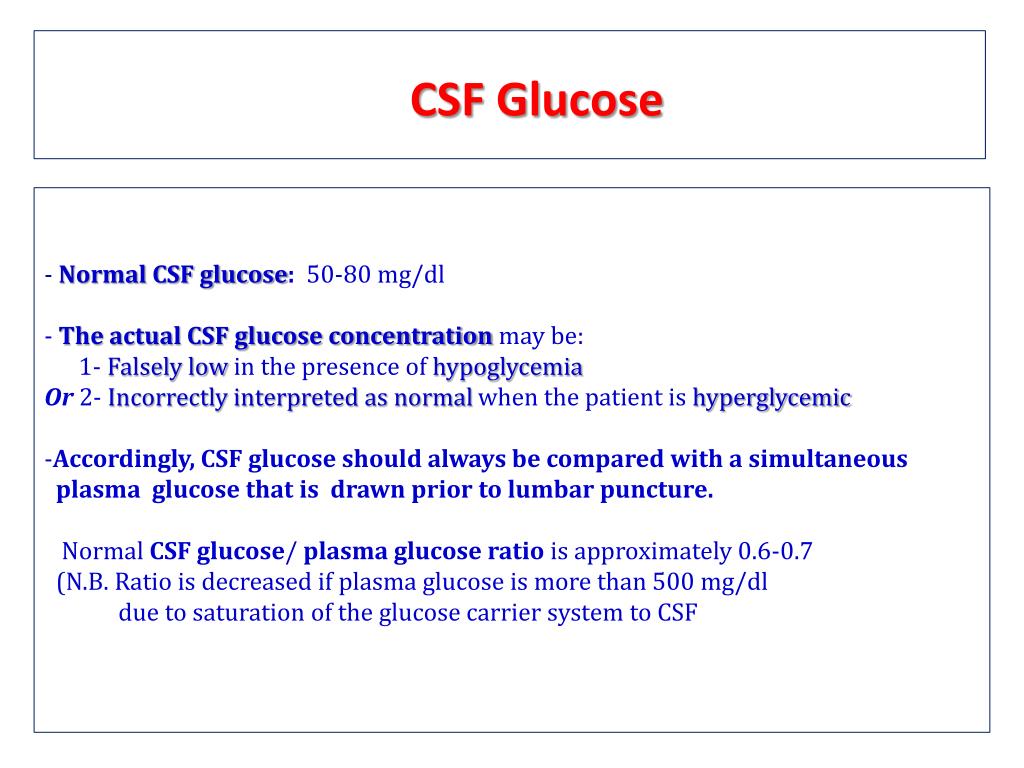
PPT - CSF ANALYSIS PowerPoint Presentation, free download - ID:3279644
 CSF Glucose - an overview | ScienceDirect Topics
CSF Glucose - an overview | ScienceDirect Topics





















![PDF] Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. | Semantic Scholar PDF] Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0ff234eca141e30a3adaa7d7e74b3379fc78dadd/3-Figure1-1.png)








Posting Komentar untuk "high glucose in csf"