herpes cure 2018 theravax
 I Met With A Theravax Herpes Vaccine Patient And Here Is What He Said | American Council on Science and Health
I Met With A Theravax Herpes Vaccine Patient And Here Is What He Said | American Council on Science and HealthRecent discoveries in the Herpes Cure is overtaken by the Covid -19 Search for a vaccineThe lack of coverage has left millions infected with herpes in the dark. Sydney, Australia, July 07, 2020 (GLOBE NEWSWIRE) -- The race is looking for a COVID-19 vaccine, the disease caused by the new coronavirus. With an estimated 9 million cases and nearly half a million deaths worldwide, the virus remains a powerful threat and the global scientific community is consumed by stopping its spread. Sinovac Biotech, headquartered in Beijing, says he could have a vaccine ready for autumn. The American Modern Biotechnology Company is currently in a Phase III clinical trial for its vaccine. And many experts speculate that the FDA could pass at least one vaccine at the end of the year. Those who know the development of vaccines know that these companies are against the huge odds. In fact, it may take 10-15 years to develop a vaccine, test its effectiveness, get approval and distribute it widely. Completing all this is in just 5 years is optimistic; crossing the finish line in less than a year is little listened. However, scientists are collaborating across borders on a scale never seen before, and many typical protocols and regulations are being trimmed or lifted to help a rapid discovery. While this is a source of hope for many communities devastated by COVID-19, the vaccine development career has shifted the attention of an area where we have already seen a major medical advance – there is finally a cure for the herpes simple virus. A holistic treatment that works For decades, researchers and scientists have worked tirelessly to find a cure for herpes and only one company has accomplished. Synergy Pharmaceuticals, a multinational organization with operations in Australia and Singapore, developed its five organic ingredients: L-Lysine, Tribulus, Astragalus, Rhodiola and a Unique Amino Formula. These ingredients work in tandem to generate T cells and macrophages, which help break down the viral envelope of herpes. This weakens the virus, prevents cell infection to cells, and eventually eradicates it. In Synergy's, 80% of the genital herpes subjects who took the Herpes Combination Treatment for 4-6 months had negative results of herpes blood tests (both HSV-1 and HSV-2). These subjects had previously been infected for years and now had no outbreaks or other herpes-related symptoms. The follow-up blood tests showed that there was no reoccurrence of the virus. In short, they had been healed. Herpes Combination Treatment was more effective than any antiviral medication currently on the market and achieved what many vaccine developers had not done. Synergy pharmaceuticals had achieved the unthinkable: a truly holistic treatment for a virus. without another known cure. This was exciting news for millions of herpes who suffer, but not for companies in the vaccine business. The Vaccine BusinessThe recent years have seen some high-profile attempts but no success of herpes vaccines. Genocea's drug testing program was blocked in 2017. The Vical Incorporated study failed in 2018. And the rational vaccines, founded by the late professor at the University of South Illinois William Halford, have been the subject of controversial and criminal investigations into their initial clinical trials. Therefore, Synergy's progress should have been acclaimed as an achievement, one that many patients had been waiting for. But that wasn't the case. Vaccines are large businesses, with the global market valued at $59.2 billion. Of course, they have enormous medical importance, as they have helped suppress or eradicate infectious diseases such as measles. And when certain populations leave or do not have access to vaccines, we typically see an increase in new infections. But vaccines are also coveted because they generate income, often for a few key corporate giants. Take the measles, for example. In the United States, Merck is the only company authorized to offer a vaccine for the disease. As a result, sales of their three vaccines – ProdQuad, MMR II and Varivax – exceed $1.4 billion per year. Imagine if another company violated its market share with a viable holistic option that worked more effectively. Merck would probably do everything in his power to protect his gains, rather than provide patients with more options. This is perhaps why Synergy's cure for herpes has not received widespread attention. In addition to taking back COVID-19 coverage, the Herpes Combination Treatment threatens to eat in the benefits of herpes antiviral drug manufacturers and any company hoping to find and sell a vaccine. It is likely that the competition will be relieved that reports on Synergy's innovative work have been buried in the news. However, this important development must be celebrated and shared. Ultimately, this type of medical discoveries are not about the company's income – they are about patients. By deleting this information, herpes patients from around the world are denied their right to a happy and free life of outbreaks. Media details – Company: Pharmaceutical Synergy CEO: Simon AndersonContact: info@synergy-pharmaceuticals.comInternet: Phone: (0)38-397-2300 Media details – Company:CEO:Contact:Internet: Phone number: Related articles Pharmaceutical synergy Sydney, AUSTRIA synergy-pharmaceutical.png Available formats:

Rational Vaccines Continues to Battle Herpes, This Time According to the Playbook | American Council on Science and Health
Americans May Get Access To Theravax Herpes Vaccine In Mexico | American Council on Science and Health
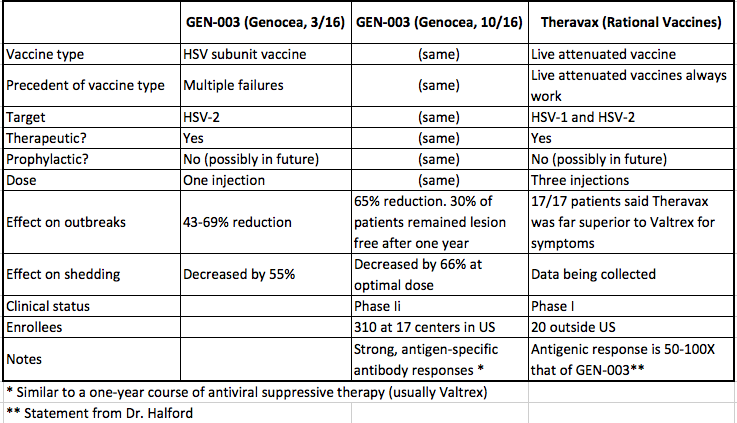
Herpes Vaccine Update: Gen-003 Phase II Results | American Council on Science and Health

Breakthrough Vaccine Could Cure Herpes In 2017 | Mohca.us

The Herpes Vaccine That Actually Works | Theravax | Heal Cold Sores - YouTube
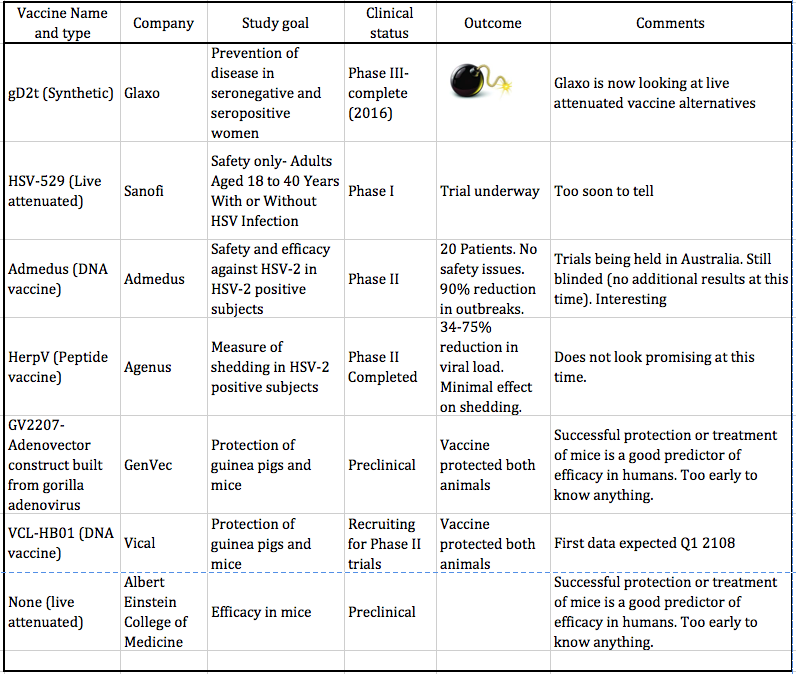
Herpes Vaccines- What Else Is Out There? | American Council on Science and Health
Genital Herpes Infection Drug Pipeline Study, H2 2018- Analysis of Phases, Companies, Mechansim, Trials, Current Status of Pipeline Drugs for Genital Herpes

Theravax 2018

Hopes for a Herpes Vaccine Have Faded

Bombs Away: Genocea's Herpes Vaccine Goes Down In Flames | American Council on Science and Health

When Will a Herpes Vaccine be Available? - Nanalyze

New herpes vaccine Theravax could end infection and suffering
First Ever Human Trial of a Live Attenuated Functioning Therapeutic Herpes Vaccine - Drug Discovery and Development

Another Herpes Vaccine Bites the Dust (Sort of) | American Council on Science and Health

One dead doctor's journey to cure herpes and say 'F-you' to the FDA | Rooster Magazine

Herpes Simplex Research - Herpes Cure Trials
Herpes Vaccine Update: An Interview With Penn's Dr. Harvey Friedman | American Council on Science and Health

When Will a Herpes Vaccine be Available? - Nanalyze

When Will a Herpes Vaccine be Available? - Nanalyze

NanoBio's Herpes Vaccine Candidate Enters The Scene — Maybe Without a Needle | American Council on Science and Health

Late researcher's work on potential herpes vaccine was promising, but he used risky human trial with no oversight
Herpes Vaccine From Down Under: Not Much Excitement For Admedus | American Council on Science and Health
A Dying Scientist's Rogue Vaccine Trial | WIRED

When Will a Herpes Vaccine be Available? - Nanalyze
Herpes Cure Secrets Natural Treatment for Hsv Virus

The Story of Lynn and The Vaccine For Herpes, Theravax.

Herpes Vaccine Shows Promise | Population Health Learning Network
Genocea's Herpes Vaccine Update: An Interview With Chip Clark, CEO | American Council on Science and Health
Herpes Vaccine Update: Gen-003 Phase II Results | American Council on Science and Health

Herpes simplex research - Wikiwand
Press | Asking for a friend

Early Tests Indicate Possible Breakthrough In Fight Against Herpes – CBS New York

News Archives - Herpes Viruses Association

Healthcare & Health solution: Herpes Cure Update 2019

Rational Vaccines Continues to Battle Herpes, This Time According to the Playbook | American Council on Science and Health

Company linked to late SIU scientist planning test of herpes treatment in U.S. - News - The State Journal-Register - Springfield, IL

More on Vical's Herpes Vaccine: An Interview with Larry Smith, Ph.D. | American Council on Science and Health

My name is Lynn and I have Genital Herpes.
Posting Komentar untuk "herpes cure 2018 theravax"