 How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling Stone
How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling StoneWarning: The NCBI website requires JavaScript to operate. Extended use of facial masks during the COVID-19 pandemic - Thermal conditioning and disinfection of the sprayed surface Mathew C. CelinaaSandia National Laboratories, Organic Materials Science Dept.1853, Albuquerque, NM, 87185-1411, USAEstevan MartinezaSandia National Laboratories, Organic Materials Science Dept.1853, Albuquerque, NM, 87185-1411, USA Michael A. OmanabSandia National Laboratories, Aerosol Characterization Team in Depts. 6633/6775, Albuquerque, NM, 87185- 1104, USAAndres SánchezbSandia National Laboratories, Aerosol Characterization Team in Depts. 6633/6775, Albuquerque, NM, 87185- 1104, USA Dora WiemannbSandia National Laboratories, Aerosol Characterization Team in Depts. 6633/6775, Albuquerque, NM, 87185- 1104, USAMatthew TezakbSandia National Laboratories, Aerosol Characterization Team in Depts. 6633/6775, Albuquerque, NM, 87185- 1104, USATim R. Dargavillec Institute of Health and Biomedical Innovation, Science and Engineering Faculty and the Centre for Materials Science, Queensland University of Technology, Brisbane, Queensland, 4000, Australia The current COVID-19 pandemic has resulted in world-wide limited supplies for face masks and personal protective equipment (PPE). Production capacity is limited in many countries and the future course of the pandemic is likely to continue with the shortage of high-quality masks and PPE in the foreseeable future. Therefore, the expectations are that they mask reuse, widespread wear and similar approaches will improve the availability of personal protection measures. Repeated thermal disinfection could be an important and probably easier option to implement in some situations, at least on a small scale, than UV lighting, irradiation or exposure to hydrogen peroxide vapor. An overview of thermal responses and continuous filtration performance of multiple types of facial masks is provided. Most masks have adequate material properties to survive some cycles (i.e. 30 min disinfection) of thermal exposure in the 75° Régimen C. Some are more easily affected, as seen by the plastic lining fuse or the gear, since the preferred conditioning temperatures are close to the soft spot for some of the plastics and fibers used in these masks. Therefore, proper temperature control is equally important. As a guide, sprayed disinfectants through diluted solutions maintain a surface presence over time extended to 25 and 37°C. Some alcohol-based solutions containing disinfectants were gently applied to the upper surface of masks. Neither moderate thermal aging (less than 24 h to 80 and 95°C) nor the smooth application of surface disinfectant sprays resulted in a measurable loss of the performance of mask filters. Subject to biomedical concurrence (additional tests for virus death efficiency) and the use of low-risk non-toxic disinfectants, such strategies, either individually or combined, offering additional antiviral or short-term refreshing properties, can complement reuse options for professional masks or face masks made now ubiquitous with their often unknown filtration effectiveness. Figure 1. Introduction The outbreak of COVID-19 is exhausting hospital and general response resources in many countries. A supply problem that has attracted global attention is the personal protective equipment (PPE), in particular by filtering the facial respirators (FFRs; the desirable type N95 or face masks widely used), which are suitable for the filtration of air pathogens and used by millions of health professionals and public personnel. N95-type masks are made by various manufacturers under different commercial names. The term 'NIOSH N95' refers to a filter efficiency rating which means that mask materials block about 95% of particles that are 0.3 μm in size or larger. PPE options and the availability of masks are likely to remain difficult, as the current divergence in medical and public responses, as well as the different apparent strategies and local availability among G20 countries and the rest of the world often with less resources. This means an additional alternative, perhaps only temporary solutions should be established for the reuse, updating or extended use of facial masks. This could reduce competition for new manufacturing masks and PPE, and also allow resource optimization for countries with limited access to primary supplies. For the use and reuse of masks, we expect continuous differences between large-scale institutional approaches, ideal strategies for hospital environments, and methods that can be quickly applied locally by non-experts with limited resources, for example the widespread use by the general public of improvised home masks. One of these options is the thermal sterilization based on multiple reports of the successful deactivation of viral material in the 70-75° Regimes C with less than 30 minutes of exposure, but with relative humidity perhaps also being a beneficial factor []. However, the survival and reliability of multiple types of facial masks and their continuous filtration efficiency under thermal conditioning is less well known. In this study and discussion, we want to address some simple questions and provide guidance to the community dealing with mask and PPE availability for the COVID-19 response, which touches on underlying aspects of material science []: as well as: 2. Options for reuse of facial masks and PPE – currently applied or under study sterilization methods After the H1N1 epidemics, H5N1 and SARS-CoV-1 there were a series of journals and reports that researched methods of disinfection/sterilization and viral matter retention for FFRs [, , ]. These studies recognized that in a pandemic the current manufacturing capacity and the vast stocks of FFR (89 million per month in the US) would have to be complemented by the reuse of FFR. In fact, the current scarcity of FFR in 2020 in many countries takes precedence over the shortage of spots experienced during the flu outbreak between 2009 and 2010. Pre-COVID-19 research and the recent 2020 research on FFR reuse offers a series of strategies for disinfection, each with its own advantages and disadvantages. Similarities in the chemical and physical stability of the SARS-CoV-2 virus to other viruses allow the assumption that the methods used in the H1N1 FFR decontamination study, H5N1 or SARS-CoV-1, can be successfully used towards SARS-CoV-2 []. This reasoning is validated by the first emerging research of SARS-CoV-2 stability that tentatively show that it can be eliminated with the usual disinfectants, UV-C irradiation, or high temperatures [, , , ]. It should be noted that the substrate can also play an important role. Chin et al. showed that after two days no infectious SARS-CoV-2 was detected in fabric, but in a surgical mask significant levels were detected after 7 days []. Given the preliminary nature of these studies, much more remains to be investigated and validated, including the investigation of all methods of decontamination to cover the resources available to disparate FFR users. High-energy irradiation has a long history as a method for sterilizing medical devices. It is known that energy photons cause degradation of nucleic acids, therefore it is effective in inactivating pathogens. It has been shown that the gamma irradiation of the FFR to 10 kGy (1 Mrad) has little effect on the adjustment of the masks (evaluated using the saccharine apparatus adjustment test), however, the filtration of particles of 0.3 μm was compromised []. Gamma radiation is also known to cause the degradation of polyolefins generally in the ю50 kGy range with dependence on the dose rate subject to an oxidative sensitivity [, , , ]. Only for masks here, the formation of radiation-induced load carrier that affects the electroret filter layers [] could be a variable that helps reduce the performance of FFR filters. Access to gamma radiation sources capable of reaching kGy doses is also not widespread, so irradiation cannot be the most convenient option for large-scale FFR decontamination. Ultraviolet radiation (UV) in the UV-C region of the electromagnetic spectrum is another form of ionizing radiation and has a long history of use on sterilizing surfaces. Unlike gamma-radiation is readily available in most of the hospital and laboratory environments. Several studies have highlighted the potential of UV sterilization of common 254 nm lamp sources [, , ] including the use of biosafety laboratory cabinets [] and a high-performance process including steps for FFRs tracking []. However, there might be aspects of the vision line, penetration for deeper disinfection (UV-C is much less penetrating than gamma) and potential surface degradation problems, as polymers are relatively sensitive to UV-C (similar to gamma doses). Hydrogen peroxide is a strong oxidant and is effective as a disinfectant either as a gas, steam or solution plasma. For FFRs, the use of hydrogen peroxide vapor (HPV) is well documented to not affect the adjustment or filter performance [,] and is promising for high-power disinfection. This is a technique that has been tested in the FFRs inoculated with SARS-CoV-2 and has been proven effective, although the results are only preliminary []. It is currently being used in some parts of the United States to disinfect large lots of FFRs for redistribution to users [] with detailed descriptions on how to implement HPV []. However, it has been shown that hydrogen peroxide gas plasma affects filter performance relative to a standard sodium chloride penetration test, although no mechanism was proposed []. Ethylene oxide gas (EO) is widely used in medical device and food industries as a sterilization method and suitable for large-scale operations. The gas itself is toxic and explosive, so attention is needed when handled and the absence of residual gas must be confirmed after treatment []. In addition, a study on residual chemicals in FFRs after the treatment of EO showed the presence of toxic reaction products that were believed to originate from the reaction of rubber straps []. Other less conventional methods for decontamination include a new methyldioxyrana oxidant mixture [], high-energy plasma electrodes generating as part of a mask [], and masks impregnated with copper oxide []. These strategies may be useful in the future, but they are not yet tested approaches. The use of heat to decontaminate FFRs is an approach that requires minimal resources as furnaces are often readily available. However, microwave heating is the exception, as it is known that it causes the deformation of FFRs, perhaps due to more unequal local heating []. The previous work with H1N1 and H5N1 have shown that 70°C to 85% humidity for 30 min [,] is sufficient to inactivate the viruses in FFRs []. Chemistry-based sterilization is easily achieved by contact with alcohol (ethanol or isopropanol) or the most classic antiviral and antibacterial compounds that can be commonly found in many commercial products. It is important to distinguish between the momentary disinfection offered by contact with alcohol and evaporation, and lasting effects that can be provided by less volatile active compounds that remain on the surfaces. The hand sanitizer usually has only isopropanol or ethanol as the active compound, while a disinfectant spray usually has a low amount of an additional active component. Antiviral properties are given by compounds such as benzalkonium chloride [], chlorhexidine gluconate [], or ionic substances such as citric acid or perhaps even EDTA, and probably also some anionic or nonionic surfactants that in essence have the ability to interrupt viral envelope []. There is usually little distinction between its engulfed activity (SARS-CoV-2 causing COVID-19) or non-developed virus (e.g., the MS2 bacteriophagus simulator), except for some comments it has been made that MS2 could be more stable than SARS-CoV-2. Curiously, the citric acid-based damping solution containing nasal sprays was cited to reduce the thyter of a strain of flu A Sydney/5/97 (H3N2) by up to 3 trunks after 1 min of contact [,]. Similarly, some oils, such as tea tree oil or thyme oil are associated with good antiviral properties []. For any use in masks and surfaces that are easily touched, it is of course important to use disinfectants that are low in toxicity and are ideally found in the existing daily items. Benzalkonium chloride is a common antimicrobial substance still on the FDA list for hand sanitizers [] and is also used in commercial disinfectant aerosols, and citric acid or tea tree oil are part of the consumer products. Again, the idea that the masks could be simply sterilized by soaking in alcohol or combined solutions (alcohol more active secondary component). However, there is evidence that some of the N95 commercial masks suffer in their filtration efficiency when they are "remodated" with disinfectant solutions based on alcohol or IAPA, or even under higher temperature steam environments []. This is due to its complex construction in terms of a hydrophobic or electrostatic layer that is relatively easily compromised in the presence of concentrated steam and alcohol solutions. However, we could also speculate that the masks might only be superficially sterilized with a carefully applied thin deposit of an active ingredient left behind an alcohol solution, basically a higher surface treatment instead of a deeper soak. Such an approach could be considered as the most basic momentary refreshment of a mask for prolonged use or short-term reuse if the conditions require such action; in principle no different from the frequent use of the disinfectant hand. Of course, any disinfection will probably be limited to the immediate surface and will not necessarily kill viral material trapped deep within the filtration layers. In addition, it would be necessary to check whether the proper efficiency of the filtration is maintained, and if a treated surface has, in fact, the additional antiviral property provided. We speculate that this will be less worrying for simple cotton or fabric masks that are now often seen with the approaches "doing it yourself". For these more lenient surfaces and similars, the reflexive application of IAPA-based solutions containing some longer antiviral compounds can be a way to increase the beneficial nature of such facial masks. The active ingredients must be selected on the basis of a toxicity or low and proven compounds already used with human contact, that is, antivirals established. Any approach to refreshing/sterilizing facial masks or enhance custom made fabric masks with the application of small amounts of spray solution that offers additional disinfectant properties, also requires that we consider how long active compounds can persist on the surface of the mask. ATR Spectroscopy (Full Attenuated Reflection) is an excellent method to test how quickly thin layers can disappear in the micrometer range or can be retained. The exact antiviral effectiveness of specific compounds must be corroborated on the basis of existing literature data, through additional or more established trials by our biomedical and virological colleagues, and is beyond the scope of our current study. Instead, we want to show how low amounts of active ingredients can show unexpected volatility or tend to remain on the surfaces for long time in RT and 37°C. This is an essential basis to further consider the use of diluted alcohol-based disinfectants, not only as the simplest method for sterilization of fast mask surfaces, but also applicable to other PPE surfaces or shared equipment, keyboards, computer mice or similar. Existing methods of sterilization and reuse, or the alternatives that are currently being considered for masks (respirators of the front piece of filling), will have to be evaluated on their own specific merits, with sterilization efficiency, filtration function and general performance (mechanical properties and adjustment) to be evaluated in parallel. This study wishes to provide more guidance for thermal disinfection approaches for most N95 masks, disinfection by applying diluted spray solutions and retention of disinfectants on surfaces.3. Thermal exposure as a simple sterilization method3.1. ApproximationIn addition to a limited supply of facial masks, a preliminary detection study consisting of a set of multiple masks was sequentially aged in slightly ventilated air lab furnaces with dry circulars for 24 h to 65°C, followed from 24 h to 80°C and 24 h to 95°C. All masks remained in excellent condition after 24 h to 65°C, however with some evidence for the initiation of material weaknesses during the final step of 95° Exhibition C. It became evident that higher temperatures or prolonged times would certainly be pushing the reliability of the mask into a unfavorable regime. A new set of masks was aged 24 h to 80°C, that is, slightly above the target sterilization temperature range of 70–75°C, and another set to validate the additional robustness for 24 h to 95°C. These two sets were inspected for signs of visual degradation while elastic bands were tested in tensile mode stretching a specific length (75 mm) per 100% (folding its length). It is important to note that the aging conditions here implied a low relative humidity simply generated by the warming of the ambient air in the ovens. There is evidence that moisture will help to deactivate the virus in stainless steel at high temperatures (up to 65°C) [], however, in the current study the optimal conditions of thermal and relative humidity for the efficiency of viral death were out of reach. Our approach is rather in the aging behavior of materials for which thermal oxidation and perhaps contraction or other immediate deformation could be important. Additional relative humidity is not expected to adversely affect polyolephins and related materials because they absorb very little water and are not sensitive hydrolytic, but the efficiency of the mask filtration and the load carrier capacity can be impacted by moisture [,]. The most appropriate thermal exposure conditions should be derived on the basis of balancing the needs of primary viral disinfection with aspects of thermal material aging, performance of mask filtration and proper behavior after exposure, and availability of dry heat, incubation chambers controlled by commercial climate, or perhaps sealed furnaces of relative humidity through aggregated salt solutions (delicence) [,]. From a material aging point of view, particularly here for polyolefins, exposure conditions of no more than one day in the temperature range of 75–95° C is not expected to result in significant oxidation, even if low-cost and weakly stabilized commercial materials are involved [,]. In addition, apart from an immediate response such as shrinking or shrinking (some copolymers have lower softness points), the thermal aging of polymers is often also of a cumulative nature, which means that a total exposure condition may be composed of multiple successive exposure intervals [,]. This means that if a material can easily handle one day at 80 or 95°C, then one would expect some repeated 1 h exposures to such temperatures to be no worse in terms of overall material performance [,]. The point is that the thermal tests for one day give confidence in the robustness of the material and that several steps of repetitive short sterilization should be achievable. We got facial masks (summarized in and shown in ) that are commonly found in the U.S. health system, and an example that is more suitable as a powder mask N95 for work under particle conditions (Moldex N95). Common-purpose mask production materials are polypropylene and polyester tissues, usually like melted or electrospun fibers []. , They offer an overview of the layer structure of such facial masks and polymer fabrics used as analyzed by the IR spectroscopy that shows spectral absorption versus wave number for material identification purposes. Commercial PPE masks cover similar filter materials (mostly polypropylene and polyester) and design principles. Therefore, any conclusion about your thermal aging and filter performance behavior is likely to be more widely applicable. Table 1Masks used for controlled aging (screen and 24 h to 80 and 95°C exposure) and subsequent filter assessment. Refuses to limited availability the Moldex mask only had a cumulative age, but was not displayed separately to 80 and 95 °C. acceptvisual checks only.ManufacturerModel3MN95 18603MN95 1860S (small)3MAura 1870+ N95Kimberly-ClarkN95 46767 facial level Layer structure of the type 3M 1860 N95 mask. Structure mask type Kimberly Clark 46767 N95. Mask type layer structure 3M Aura 1870+ N95 using only PP-based materials. 3.2. Observations after the 24-hour exposure to 80°C and 95°C shows a set of thermoconditional specimens including a facial shield that clearly shows the weakness of a molded PET sheet. Likewise, shows thermal air conditioning at 95° C with more evidence for the reduction of the level of the Halyard fluid shield 3. , show more details of weaknesses in some types of masks that could be visually recognized after thermal exposure. shows some of the observed fissures for the type of Moldex mask and its ventilation deformation at 95° C.The different masks age thermally for 24 h to 80°C before and after exposure. Different thermally aged masks for 24 h to 95° C before and after exposure. Thermally ages for 24 h to 80°C before and after exposure. There is some adherence between the two layers for the Kimberly-Clark N95 mask (on the left). More pronounced escape of a specific type of 'duckbill' mask (Kimberly-Clark 46767 N95) with these images taken in the final stage after the cumulative upbringing of 24 h each to 65, 80 and 95°C. Moldex mask cumulatively aged for 24 h to 65°C C and 80°C showing a functional edge of phyll (high but red in appearance), After the additional exposure of 24 h to 95° C the ventilation assembly in the center was partially deformed (blanqueamiento) with additional crack at the bottom of the mask. 3.3. Summary of 24-hour exposure to 80°C, i.e. moderate ageing 3M 1860/1860S – The aged mask appears almost identical to the original specimen not aged. It has the same color, but the printed ink on its forehead has been slightly diffused in the fibers (press of cherry). Nose and elastic foam bands are still intact, as elastic bands retain their initial stretching properties. Elastic bands seem durable and can easily support the prolonged exposure of 80°C without obvious degradation. 3M Aura 1870+ N95 – This mask keeps its color, there is no obvious diffusion of ink, and elastics retain their original ability to stretch. The foam of the nose stays attached safely and there are no apparent problems with this mask, so easily accommodate this 80° C exposure without obvious degradation. Kimberly-Clark N95 – This mask keeps its color, but there is a certain diffusion of ink, reduction and partial marketing to 80°C. The top of the 'duckbill' has a metal band embedded to mould the mask to the user's nose. The bottom part doesn't have the metal band and the plastic there is. This could affect the adjustment in the chin and could allow the particles to deviate from the chin seal. In addition, the inner plastic lining (see ) appears partially merged, and does not allow the 'duckbill' to open easily more without tearing the inner polymer lining of the filter sections (based on IR it is likely that some ethylene copolymer with acrylate methyl). This inner lining would have to be separated while in the oven. It is also possible that the use of the mask can prevent any subsequent adhesion, as the surfaces can no longer touch with the most effective. It can help the mask open in its 'duckbill' configuration before thermal conditioning (not flat and folded). For the exhibition, here, we prefer not to open the mask to get a better image of the intrinsic filter performance. Likewise, other masks or objects should not be stacked at the top to prevent this inner coating from coming in contact and fuse. The elastic bands made with sufficient remaining elasticity. Halyard Fluid Shield Level 3 – This mask maintains its color, but there was a certain diffusion of ink, reduction and partial control at 80°C. In contrast to the Kimberly-Clark model, the lower part of the 'duckbill' does not have the metal band and the plastic is armored. Again, this could affect the adjustment of the chin and allow particles under the seal. Since this mask does not have the inner plastic coating, there is no problem with any cast polymer lining due to a lower fusion material. Again, elastic bands had enough remaining elasticity. MediChoice Facial Shield – This polyester (PET confirmed by IR) face screen shows a significant reduction and was completely destroyed. He can't stand 80°C for 24 h. We note that the foam on the forehead and the elastic band was still securely attached, and there was no problem with foam and elastic. This facial shield also does not support shorter times and slightly lower temperatures (i.e. 30 min to 75°C). Moldex 2400 N95 – Only one mask was available as part of the initial cumulative detection exposure from 24 h to 65, 80 and 95°C. This mask maintains its color and did not notice obvious changes after initial aging to 65°C. After ageing at 80°C for 24 h the lower edge of the mask began to break slightly. Again, this could affect the chin adjustment and could allow particles under the seal. The performance of the elastic band retains its initial properties of stretching after this cumulative aging schedule. Ageing at 95° C for 24 h is too much for this mask as significant structural failures were observed. The cut in the chin section was more pronounced, and the high-impact polystyrene tube (confirmed by IR) began to deform and warp. Exhibitions for 24 h to 95 °C: Similar observations apply to all the masks described above for 80°C. This means that these masks and their polymer materials have a somewhat preponderable behavior for high temperature exposure. Types 3M 1860, 1860 and Aura 1870+ N95 are not expected to significantly degrade if they experience temperatures slightly higher or higher than the lowest sterilization targets. These masks have some intrinsic thermal robustness. In contrast, unfortunately, the types of "duckbill" (Kimberly-Clark 46767 N95 and Halyard Fluidshield Level 3) have a specific material vulnerability with some aviators as a material response, which could compromise their best subsequent adjustment. In addition, there is the problem of potential adherence between the two layers for the Kimberly-Clark N95 through the additional lining. We encourage any end user to check for this behavior and its impact should be applied thermal sterilization. In short, 3M 1860, 1860s, together with the Aura 1870+ N95 show better thermal performance. Based on the results of the exposure test, these masks could be thermally treated at a temperature of 75° C and be reusable, provided that the effective viral deactivation is confirmed and its filter efficiency remains equally functional. A previous study also showed that dry thermal treatment had little influence on the performance of masks of a particular type []. The Kimberly-Clark and Halyard 'duckbill' masks showed some deformations on the chin seal that could lead to a compromised fit. This should be verified in a few specimens with individual responses of parallel adjustment tests. We also recognize that the 24 h to 80° C tests are perhaps more conservative than just a few sterilization exhibits at no more than 75°C. This means that the 'duckbill' type masks can still be performed after limited thermal exposure.4. Simple improvised solutions disinfectant for surface disinfection and expand the use of masks or PPE4.1. FocusThe large-scale institutional approaches for the sterilization of masks and PPE exist as treatment of hydrogen peroxide vapor, which has been widely reported and is now approved by the FDA [,], and perhaps gamma irradiation when the installations are readily available (but polymer materials and filter reliability is confirmed) or UV lighting [, , ]. We also recognize that aqueous solutions of isopropanol or ethanol are used in hospitals for rapid momentary disinfection, with some discussions on the web that ethanol could be more effective than isopropanol for SARS-CoV-2. While the hard surfaces are easily cleaned and can be extended to a suitable PPE, the so-called mask alcohol soaking is seen to be detrimental to the efficiency of the filtration and the performance of masks []. Once the alcohol has evaporated, there is also no extended sterilization effect on any clean surface, so cleaning with wipes is temporary at best. This raises the question of whether there can be simple improvised alternatives where long-term antiviral compounds are easily deposited on appropriate surfaces, including masks. This would offer an active surface disinfection or on request, beyond the mask being only an inert physical barrier. With the rapid spread of fabric masks made as they have an unfamiliar filtration efficiency, there may be added value for such quick refreshing options, similar to the use of hand-infectants. In this sense, we see value in improvised applications of a soft fog of alcohol solutions containing an active secondary compound. This could also be carefully applied to the perhaps vulnerable surface of facial masks, if there is no other quick disinfectant method available and the extended use is absolutely necessary. As mentioned above, COVID-19 is a global pandemic that affects many countries with undoubtedly limited opportunities for disinfection and recycling technologies for high-tech masks and PPPs. We preview simple disinfectant solutions and manual diskettes that are widely available. We also recognize that these can already exist and that they are likely to be used subject to the experience and improvisation skills in local health services and communities. They may not offer a complete disinfection, that is, deep from the mask, but they can certainly address surface pollution and help with repetitive handling. Simple compounds with known antiviral disinfectant properties are ionic surfactants, benzalkonium chloride, chloroxydine gluconate or povidone-iodine and similar [,]. These substances are used in many products, e.g. the EDTA disodium is added to freely available contact lens solutions, the chloride of sterilium is in the hair conditioner, and chloroxydine gluconate is an ingredient in oral washing to treat gingivitis or periodontal disease. They were more widely available in the past before they have attracted the additional FDA scrutiny recently with continuous examinations of active ingredients antiseptics of free sale [], however, such compounds are recognized as the key ingredients in many antiviral formulations []. Ionic species and substances with a strong hydrogen union are expected to interrupt the viral packing layer or the "docking" surfaces described as trimeric tip glycoproteins []. From an alternative point of view, tea tree oil, for example, has also been reported as having good antiviral properties when applied on surfaces [], or citric acid that has strong ionic characteristics in the low pH range that is considered antiviral []. Our intention here is not to specify the exact antiviral properties and individual pro/cons of specific disinfectants, but rather to show how such active compounds could be easily applied improvisedly and if they can remain on surfaces as very thin layers. The ATR infrared absorption spectroscopy as a surface-sensitive technique is ideal to quickly explore relative volatility and the continued presence of any deposited compound. 4.2. Deposition of very thin layers of disinfectants We use a commercially available disinfectant spray and prepare some representative solutions of antiviral/deinfectant compounds dissolved in 70 vol% isopropanol for a simple detection study. A high fraction of isopropanol has two advantages, namely, the behavior of momentary disinfection, but also a low viscosity of solution that helps the generation of a fine fog. This is more difficult to generate from solutions that are richer in water that result in larger droplets and more heterogeneous surface coatings. We evaluate the following solutions that involve surfactants, commercial disinfectants, essential oils or other ingredients that may have antiviral properties such as EDTA or copper sulfate. Povidone iodine can also be effective, but it was not obtained for the current study: These solutions were deposited with two short grids of a small bottle of spray subjected to distances of 20 to 30 cm. The spectral acquisition was initiated and monitored more than 24 h to 25 and 37°C. The isopropanol carrier evaporates quickly leaving a thin film of the active substance remaining in the ATR crystal. 4.3. Retention of disinfectants on surfaces by spectroscopy ATR After depositing a fine film of the alcohol/disinfectant solution in the ATR crystal, the immediate spectral changes showed the rapid loss of isopropanol and water, usually in the order of 10 min to 25 and 3 min to 37°C. For commercial disinfectant spraying, there was evidence of the active compound (aquatic ammonia seal) that remained for about 25°C, but rapidly disappearing to 37°C (G) ). The residue of a commercial disinfectant product (Pine-Sol, especially water surfactant) was easily maintained on the glass surface for 24 h at both temperatures ( ). In contrast, despite its attractive smell and antiviral properties, the tea tree oil dissolved in the alcohol/water mixture quickly disappeared within 1 h to 25°C (G) ). Tea tree oil may be useful for short-term disinfection, but it will clearly not remain sufficiently on the surfaces. A similar disadvantage volatility was observed for limousine as the active compound. Citric acid in combination with some small amount of surfactant, however, forms a persistent film within 10 minutes (time sufficient for the complete evaporation of the solvent carrier) that does not show any spectral change ( ). This is an example for excellent surface retention if a film deposited is not touched after the deposition. The commercial disinfectants in individual concentration of 1% are applied here as a mixture of benzalkonium chloride detection, EDTA and chloroxydine gluconate form an equally stable film deposit ( ). For visualization purposes, a thin micrometric-size film is displayed at a top of reflective bank for the citrus acid solution. This deposit is not toxic and is easily cleaned. Subject to proving that such very small amounts of residues will not interfere with the efficiency of the filtration of a mask, we suggest a new evaluation of such simple disinfectant sprayers for the refreshing and extended wear options. Basic disinfection solutions are easily improvised and therefore widely available. Of course, any option for a specific and active constituent solution will have to be evaluated on its own specific merits, with the function of disinfection of efficiency and filtration that will be evaluated. We wish to emphasize that surface retention is equally important and that relatively non-toxic disinfectants are also available. Deposition of a soft layer of spray surface of a commercial Lysol can on the ATR glass surface. There is a limited retention of benzalkonium chloride at 25°C (top) and a rapid loss to 37°C (bottom). Deposition of a soft layer of spray surface of 25% pine cleaner in 70 vol % isopropanol-agua of a portable spray bottle on the ATR glass surface. There is good surfactant retention (and probably other constituents) at 25°C (top) and 37°C (bottom). Quick loss of a sprayed surface layer of 3% of tea tree oil in 70 vol % isopropanol-agua deposited from a portable spray bottle on the glass surface ATR to 25° C. Excellent retention of a sprayed surface layer of 3% citric acid and 1% surfactant (StepWet DF 90) in 70 vol % isopropanol-water sprayed bottle. Excellent retention of a mixture of compounds in 70 vol % isopropanol-water (1 wt% gluconate chlorroxidine; 1 wt% disodium EDTA; 1 wt% chloride benzalkonium) gently deposited from a bottle of hand spray on the glass surface ATR to 25°C (top) and 37°C (bottom). Example of a very thin layer of citric acid and surfactant deposited as a fog of a small bottle of portable spray after the evaporation of isopropanol / water carrier. 4.4. Application of a diluted spray solution on mask surfaces Taking into account the overall impact of the COVID-19 pandemic we also need to consider that the latest generation disinfection and reuse approaches cannot be available everywhere. There may be no options such as hydrogen peroxide exposure, irradiation or large-scale basic thermal conditioning for some countries. In such situations, improvised approaches for reuse of PPE and masks can be limited to visual inspection, perhaps cosmetic cleaning or not more than the application of small amounts of disinfectant sprayers. As mentioned above, deep cooling with isopropanol or similar, or the actual washing of these masks is strongly discouraged as it negatively affects the filter layers electret [] and is expected to reduce the filtration performance of particles. But what is to apply a minimal surface treatment to allow a momentary "refresh" of the external surface with disinfectant? As we have shown above, isopropanol/water could act as a suitable carrier for common disinfectant and surfactant additives. In comparison, the tea tree oil spray caused a thin surface deposit that is surprisingly evaporative. However, it would also have a time of residence on the surface of the mask and help in the temporary disinfection of the surface, perhaps even through some penetration of vapors in the filter layers. We do not want to recommend a specific formulation approach or comment on the exact effectiveness of individual compounds for their viral deactivation performances on mask surfaces and their substructure, apart from known disinfectants can be applied on the outside surface and are expected to have some benefit. Our approach is the technical feasibility of spray coatings using non-aggressive ingredients with established antiviral properties. At the same time, we also believe that there are many options for suitable disinfectants along with some surfactants in alcohol/water solution. We note that in the internal efforts of SNL related to the cleaning and disinfection of hard surfaces, a combination of a surface cleaner of 3% commercial (Pine-Sol) with only benzalkonium chloride of 0.3% in water showed an excellent death efficiency for MS2 viral simulator in silver solution with a reduction of trunks greater than 5 (Let's show the retention of previous glass TRYPs. We add a small amount (droplet) of red food dye solution to our 'symfectant solution' in order to visualize the spraying tanks in masks. With a low-cost, 50 mL thin sprayer we were able to achieve a homogeneous surface layer. The solutions were prepared using 70/30 vol% isopropanol/water as a carrier with added active ingredients to allow a simple detection of how disinfectants can be deposited. A 3M Aura 1870+ The N95 mask was coated with a weak solution that contains 3% citric acid and 1% liquid hand soap resulting in homogeneous coverage (i.e. ). The Halyard mask was sprayed with 25% added of a commercial cleaner (Pine-Sol that has a non-ionic surfactant ingredient) as shown in , and the Kimberly Clark mask with a solution containing 1% Pine-Sol and 1% benzalkonium chloride (a rudimentary solution that has a surfactant trail and a known relatively easy and low risk disinfectant). A 3M 1860 mask was coated with an addition of benzalkonium 1% chloride, chlorohexidine gluconate and 1% EDTA disodium (the intention here is for basic ionic activity) for demonstration purposes only. In all cases, a thin coating can be easily applied. Of course, commercial sprayers with improved atomization features could apply thin layers more easily in multiple parallel masks. Thick films of citrus acid and surfactant are easily deposited as a fog of a small bottle of hand spray after the evaporation of isopropanol / water carrier. The solution had a small amount of red dyes to help visualize the thin distribution of films. The surface of the mask is barely covered to the left and double covered to the right. Fine Pine-Sol disinfectant cleaner films are easily deposited as a fog of a small bottle of manual spray after the evaporation of isopropanol / water carrier. The solution had a small amount of red dyes to help visualize the thin distribution of movies in a Halyard face mask. In situations where chemicals-based disinfectants cannot be available at all, then the most basic approach could be a smooth exposure of the surface of the mask to a bit of isopropanol/water weak spray or similar as a carrier with some surfactant added as an active ingredient. This is based on reports that even hand soap has some antiviral properties [], as shown in . Of course we recognize that significant disinfection in this case is probably not guaranteed, but an aerosol approach of this nature would help the mask user to minimize the superficial contamination of repetitive manipulation and superficial resident viral exposure.5. Filling performance after thermal conditioning and application of some disinfectants Efficiencies of the thermally aged and gently coated mask filtration were characterized to assess whether a discernible difference could be observed when compared to untreated N95 masks. An RT filtration system was used in the Sandia National Laboratories to quantify the efficiency of the Kimberly-Clark and Halyard masks as a function of particle size. The system simulates, where possible, the parameters defined by the National Institute for Occupational Safety and Health (NIOSH) for the certification of filtering materials for N95 respirators (NIOSH 2019) []. The relevant description of the system [] contains a detailed description of the system, the instrumentation used and the methodology applied. The complete mask geometries were mounted on the custom filter supports and inserted into the system test section. A polydispersion NaCl test spray was used, and current measurements were taken up and down from test articles with a TSI Scanning Mobility Particle size spectrometer (SMPS) 3938. Each respirator was tested at two filter-face speeds as suggested for the evaluation of the filtration performance of fabric masks and common tissue materials [], which were derived from the lower and higher flow rates defined in the NIOSH standard. Complicated measurements were made for each test configuration. The efficiency of the filtration in each size of the measured particles was composed of the difference in the concentration of aerosol up and down the test article. Care was taken to ensure that only statistically significant data were reported. Uncertainties in these measurements were thus spread through all calculations. The relative performance of mask filtration was examined in the range of 10–400 nm, that is, a range of particle size that is very important to be filtered effectively, comparing the performance of specimens treated with an untreated reference mask. Data for low and high flow conditions for the Kimberly Clark mask when treated at 80°C for 24h and sprayed with 1% Pine-Sol and 1% benzalkonium chloride (70/30 isopropanol/agua) solution are shown in . Clearly there has been little effect of the two treatments, although there are some scattered in the data subject to the limited availability of masks and our inability to test multiple facial masks. We hope that some underlying mask mask masks the variation in any way, subject to different batches, perhaps handling during the acquisition, or its relative age as well. In addition, although identical in appearance, these Kimberly Clark models were also provided with different brands depending on the suppliers (i.e., a mask had an additional Technol stamp on its rear surface). A comparison, again for low and high flow speed conditions, for the Halyard mask is shown in . This type of mask has intrinsically less dispersion and even though the spray coating is applied generously (see ), evidence is that neither the disinfectant sprayer nor the thermal exposure to 80 and 95° C had measurable consequences in the filtration performance. Relative changes in treatment filtration efficiency (termally or covered with 1% Pine-Sol and 1% benzalkonium chloride in 70/30 isopropanol/agua solution) Kimberly Clark masks in the range of particles of 10 to 400 nm low (top) and high (low) flow conditions. Relative changes in treatment filtration efficiency (such as or covered with 25% of a commercial cleaner (Pine-Sol that has a non-ionic surfactant ingredient) in 70/30 isopropanol / water solution as shown in ) Hawk masks in the range of particles of 10 to 400 nm low (top) and high (low) flow conditions. An additional filter efficiency study was performed in a separate filter penetration test (FPT) background located in SNL. As with the above system, this test bench was also designed to follow, where possible, NIOSH guidelines for the N95 breathing material test. The FPT differs from the previous system, as the size-selects a NaCl mono scatter spray using an electrostatic TSI 8020 sorter and differential mobility analyzer TSI 3081L (DMA) of a 47 mm filter housing. NIOSH guide describes an aerosol with a medium diameter of particle size of 0.075 ± 0.020 μm with a standard geometric deviation not exceeding 1.86, which the FPT system verified. However, it is worth mentioning that the monodisperous spray does not capture the diameter of medium particles recommended by the FDA of 0.3 μm. In addition, the diameter of the filter housing used by the FPT is less than the assemblies used by TSI 8130 (120 mm). Therefore, the flow rate was adjusted according to the equivalent filter face speeds, so the filter housing geometry was mitigated. Finally, to calculate the filter efficiency, the water concentration up and down of the filter material tested by a TSI 8022 condensation particle counter (CPC) was compared. Five current and downstream tests were performed with intervals of 1 min. Mask performance tests that produce relative filtration efficiency are reported below, as summarized in for two types of N95 facial masks (the 1860 and Aura 1870+ of 3M) under multiple exposure conditions. Once again, the results show that the disinfectant sprayer or thermal exposure to 80 and 95° C did not have measurable consequences on the filtration performance. Table 2Retroactive filter efficiency for two 3M type masks under multiple conditions of measured exposure with a Penetration Testbed filter (FPT) using mono-dispere particles 75 nm NaCl with a velocity of the filter face of 17.34 cm/s. The 3M 1860 mask was coated with benzalkonium chloride 1%, chlorohexidine gluconate 1% and disodium 1% EDTA for demonstration purposes only, and the 3M Aura 1870+ N95 () was coated with 3% citric acid and 1% liquid hand soap, respectively. The constituents were always dissolved in the isopropanol/water solution 70/30. Model Brand statusEfficiency [%]Stdev [%]1860Unged (reference)97.740.38186080°C, 24 h99.570.21186095°C, 24 h99.180.101860Coated98.410.08Aura 1870- + N95600. Conclusions We have focused on two directions to allow the widespread use of PPE face masks. A path that has also been recognized by others (see recent literature) is the use of thermal exposure for the disinfection of masks in the 75th Rank C, subject to confirmation of the times and temperatures most suitable by our biomedical colleagues. Another strategy, especially if local resources are limited and institutional approaches to treatment of large-scale masks are not available, the subtle spraying of the surface of a mask with a weak disinfectant solution could be an improvised option to allow some topical disinfection, or at least a refreshment of surfaces that are often touched and can be contaminated. From the performance point of a material only a few minor problems were observed during thermal exposure. Some masks showed a cover-up and the beginning of material weaknesses, but the most important thing was not observed a significant negative impact on the filtration performance due to thermal condition no more than 24 h to 80 °C or 95 °C. In addition, the filtration performance was maintained despite the application of a spray coating on mask surfaces. There is good confidence in the viability of some repetitive steps of thermal reconditioning, considering that the proven masks are mostly resistant to temperature and times higher than those currently discussed for the requirements of killing viruses. Alcohol-based diluted and soft solutions that contain low-risk non-toxic disinfectants, perhaps improvised due to lack of local availability of ingredients and when applied to the upper surface only (i.e., avoid soaking), can complement the face masks made now ubiquitous with their often unknown filtration effectiveness. Similarly, this could be a viable option for any mask and other PPE to provide additional or short-term refreshing rapid antiviral properties. We do not recommend a disinfectant formulation in particular, but rather introduce the current concept, and we wish to emphasize that the specific effectiveness of viral killing of any disinfectant on masked surfaces has not yet been determined. However, we have shown that low toxicity disinfectants can be applied to masks without affecting filtration and structural characteristics, and that benzalkonium chloride (still on the approved list for manual disinfectant []) with solution surfactant is effective against the MS2 viral simulator, which corroborates the disinfectant efficacy against COVID-19 []. In addition, we have not addressed any risk of inhalation, which due to the nature of needing any disinfectant cannot be zero. Collectively soft spraying tanks ~100 mg (for two sprayers) of material on the surface of the mask, which for example with active content of 1% means approximately 1 mg of disinfectant mixed with some surfactant could be attached amorph to the surface of the outer mask. The risk of exposure to such quantities cannot be eliminated, but it will need to be balanced against the needs of improving the availability of masks, longevity and antiviral effectiveness in the context of other options may not be available. As with many other aspects of this pandemic, careful and informed commitment to, of course, individual preferences (including disinfectant choice) should be pursued, as there are no perfect and zero risk solutions. Our results on thermal and spraying approaches represent some future opinions that should not be misinterpreted. Any use of thermal disinfection and spraying in masks should be considered within the additional risks seen in the use of chemical disinfectants and the behavior of mask adjustment after thermal reconditioning. In addition, a surface sprayer is not expected to kill virus particles trapped inside the inner filter layers. However, commercial masks cover similar filter materials and design principles. Therefore, any conclusion on your reuse and filter performance is likely to be more widely applicable. However, continuous adjustments of good mask and general cleaning are also important for prolonged use. Disclaimer These are subjective and personal opinions of researchers in the reliability of polymer materials and the biomedical field in the context of the current global pandemic COVID-19. There is no intention of challenging the existing guidelines for PPE/mask use policies or of giving preference to PPE/mask manufacturers and specific technologies that already exist or are in development. Due to the time constraints caused by the urgency of the COVID-19 situation, these screening studies should not be considered comprehensive assessments with any implicit recommendation and guidance beyond the scope of these efforts. The parallel work of other researchers is expected to complement our understanding of the PPE mask and performance. Collectively this will guide us to viable methods and approaches for reuse of masks/PPE and additional application options. SNL and QUT do not support specific approaches to disinfection of masks; we only want to report on their thermal responses. Medical experts and virologists will have to make decisions based on COVID-19 deactivation properties for the right time and temperatures to allow reconditioning of masks and other PPE. Statement of Authorship CRediT Mathew C. Celina: Conceptualization, acquisition of funds, Research, formal analysis, Resources, Visualization, Writing - original draft, Writing - review & editing. Estevan Martínez: Research, formal analysis, Visualization, Writing - original draft, Writing - review & editing. Michael A. Omana: Research, Methodology, formal analysis, Visualization, Writing - original draft, Writing - review & editing. Andrés Sánchez: Research, Methodology, formal analysis, Scripture - original draft. Dora Research, Methodology. Research. Tim R. Dargaville: Conceptualization, Scripture - original draft, Scripture - review & editing. Statement of competing interests The authors declare that they have no financial interests or personal relationships that could have influenced the work reported in this document. Allison Paul of the department of prevention and control of infections at the Presbyterian Hospital of Albuquerque is grateful for the contribution of multiple facial masks. We also want to recognize Taylor Settecerri for his auxiliary support to the experimental work of mask filtration characterization and Ann Dallman for the corresponding data analysis. Catherine Mageeny and Cathryn Siegrist are valued for their understanding and comments. We also thank Erica Redline for the supply of some sprayers and valuable comments, as well as Anne Grillet and Martin Nemer for emphasizing the nature of the electroret materials and small mask samples that allow IR analysis. T.D. is supported by the ARC Future Fellowship Scheme (FT150100408). Sandia National Laboratories is a laboratory of several missions managed and operated by National Technology and Engineering Solutions of Sandia, LLC., a total-owned subsidiary of Honeywell International, Inc., for the National Nuclear Security Administration of the United States Department of Energy under contract DE-NA-0003525. Any opinion or subjective views that may be expressed above do not necessarily represent the views of the United States Department of Energy or the United States Government. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
A cutting-edge research firm focused on digital transformation. Good. Subscriber Active account since then Exclusive audio book of subscriber! "No Rules: Netflix and the Culture of Invention"Put it now in Book.fm using the button below. How to clean your fabric face mask between trips to the shop for essential products If you buy through our links, we can earn money from affiliates. Now that the (CDC) has recommended preventing COVID-19 transmission, and with disposable masks in short supply, it is important to know how to handle and clean reusable fabric masks properly. We spoke with , an immunologist and national spokesman for whom they reiterated the importance of covering their faces in public. Popular reviews"Many people can be asymptomatic carriers of the virus, so they can have it and be spreading it without even knowing it," he said. "Therefore, protect others in case you sneeze or cough while you're talking and respiratory particles are airborne. And they should be used when they can't be more than six feet away from people." Can I clean my face mask at home? Disposable masks and filters made of non-woven fibers, KN95, and cannot be safely cleaned at home. Exposure to excessive amounts of water and cleaning products will destroy fibers and can damage the carbon filtration systems of N95 respirators. KN95 or N95 masks should be reserved for medical workers, but if you have one, you know they can be. There are exceptions if the mask is used in certain medical procedures, damaged or heavily dirty. Due to the shortage of supply chains, researchers have had to find out if and how N95 masks can be sanitized to reuse. Researchers at the University of Illinois, Urbana-Champaign found that 50 minutes of dry heat in an electric cooker like an Instant The leg can kill four types of viruses in , including a coronavirus. After preheating the kitchen for five minutes until it reaches 347 °F, use a towel in the pot to lin the inside and then place your mask over for 50 minutes. The inventor of N95 masks, , and scientists at the University of Tennessee are also studying the effectiveness of using ultraviolet-C (UV-C) light as a disinfectant. However, procedures for cleaning these masks or filters at home have not been approved. The face masks of fabric, on the other hand, can and should be washed at home. They can be reused until they are torn or no longer fit in the user's face. How to wear and remove a face mask correctly Before putting a face mask, or using. Identify the outer and interior sides of the mask. Always make sure to place the inner surface next to your skin. The mask should be used firmly against the face and cover both the nose and the mouth. Try not to touch the mask while wearing it. When it is time to remove the mask, slide the elastic bands from your ears, or untie the strings. Do not grab the front of the mask to remove it from the face. Using elastic strings or bands, place the mask directly into a paper bag for removal, or hold it until you are ready to wash or wear it. Wash your hands again. How to clean a mask from the face of the fabric. Elliott recommends after every use. It is a good idea to have several hand masks that can be turned, so a clean is always available. If you need to buy some extras, here they are, , , and our favorites . Clothes masks can be washed machine or washed by hand. By manipulating dirty masks, it is a good idea to wear disposable gloves and keep the masks away from your face. How to disinfect fabric masks In my years of testing different cleaning methods in fabrics in a textile lab, I learned that washing cotton in hot water and drying it in the high heat environment will effectively heal it. However, if someone in your home is sick or has a compromised immune system, you may want to add a disinfectant to the washing cycle when cleaning tissue masks to ensure germs are killed. The type of disinfectant you should use depends on the tissue of your mask. While the chlorine of bleach is an excellent disinfectant, it can weaken and discolor fabrics if used incorrectly. Always read product labels and carefully follow instructions. Due to the new coronavirus, some of these products may be available online or only available in the store. We've decided to keep the links since we've seen availability coming in and out. Types of disinfectants to use Get the latest from Business Insider Intelligence on how COVID-19 is affecting industries. Disclosure: This post is brought to you by the team. We highlight the products and services you can find interesting. If you buy them, we get a small portion of the sales revenue from our business partners. We often receive free products from the tested manufacturers. This does not drive our decision on whether or not a product is presented. We operate independently of our advertising sales team. We welcome your comments. Send us an email. Was this article helpful for you? Popular reviews

How to Reuse and Dispose of Your Masks Safely | the Beijinger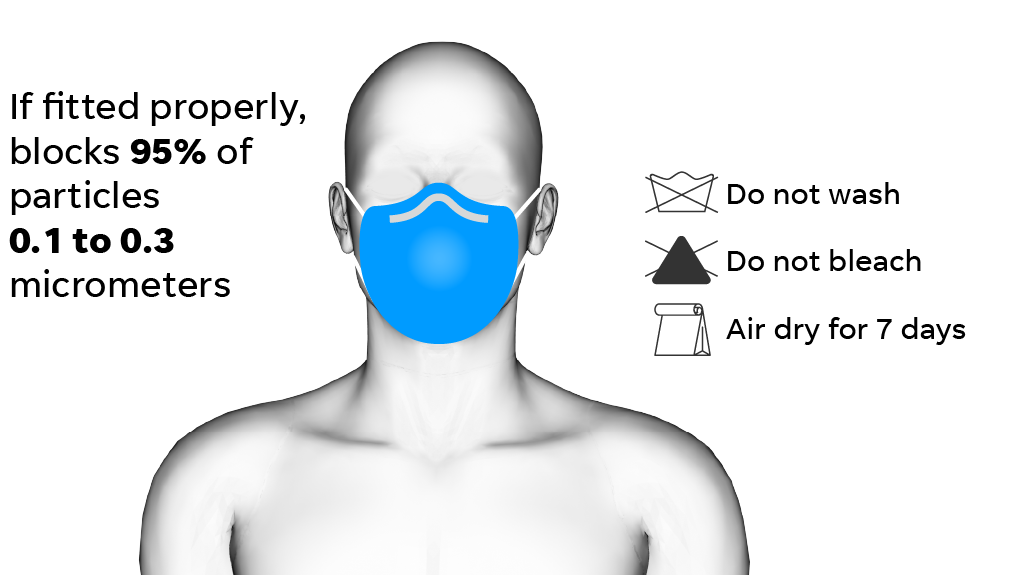
Coronavirus masks: How to clean, sanitize and reuse your mask
How Do I Clean And Maintain A Reusable COVID-19 Mask? : Goats and Soda : NPR
How to clean or sanitize a face mask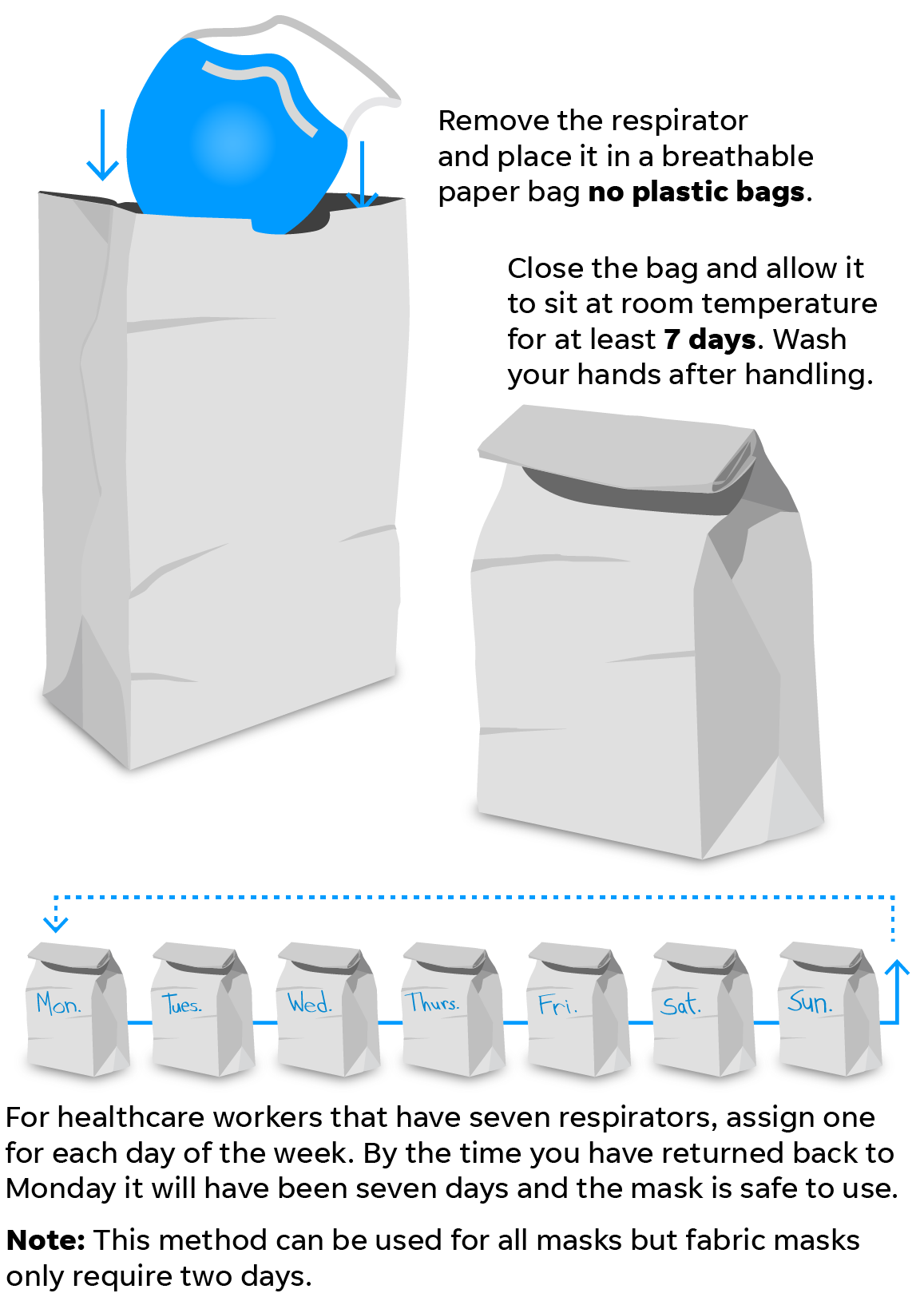
Coronavirus masks: How to clean, sanitize and reuse your mask
Can disposable face masks be reused? - Chinadaily.com.cn
Should I Purell My Nostrils? Can Lysol Disinfect The Air? : Goats and Soda : NPR
How to clean and sanitize your cloth face masks - Chicago Tribune
Why the best material for a homemade coronavirus face mask is hard to identify
Should I Purell My Nostrils? Can Lysol Disinfect The Air? : Goats and Soda : NPR
Private tech companies mobilize to address shortages for medical supplies, masks and sanitizer | TechCrunch
Is Washing Masks Effective After Virus Exposure?
How to Disinfect Everything: Coronavirus Home Cleaning Tips | WIRED
Coronavirus: Who needs to wear a mask and what's the proper way to wear it?, Singapore News & Top Stories - The Straits Times
Face Mask Cover Guidelines for Homemade Cloth Masks | University of Utah Health
How to Disinfect Everything: Coronavirus Home Cleaning Tips | WIRED
Video shows Taiwanese why spraying alcohol ruins masks | Taiwan News | 2020/03/09
Coronavirus masks: How to clean, sanitize and reuse your mask
Duke University uses vaporized hydrogen peroxide to clean N95 face masks for reuse | TechCrunch
How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling Stone
Extended use of face masks during the COVID-19 pandemic - Thermal conditioning and spray-on surface disinfection - ScienceDirect
A Study Looks at How to Disinfect Your Mask at Home - Coronavirus Coverage
How to Clean Cloth Face Masks at Home
Makers of Wipes and Hand Sanitizers Step Up Production as Coronavirus Spreads - WSJ
How Do I Clean And Maintain A Reusable COVID-19 Mask? : Goats and Soda : NPR
Extended use or re-use of single-use surgical masks and filtering facepiece respirators: A rapid evidence review - The Centre for Evidence-Based Medicine
How To Clean Your N95 Mask Or Face Covering With Covid-19 Coronavirus
How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling Stone
Face masks: The most purchased masks we've recently covered
Lysol, Clorox sprays and wipes say they fight human coronavirus? Why?
How to Disinfect Your Mask: A Step-by-Step Guide - Coronavirus Coverage
Wash your mask daily: the ultimate guide to face coverings | World news | The Guardian
Coronavirus face mask cleaning and reuse: What you need to know - CNET
Why the best material for a homemade coronavirus face mask is hard to identify
How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling Stone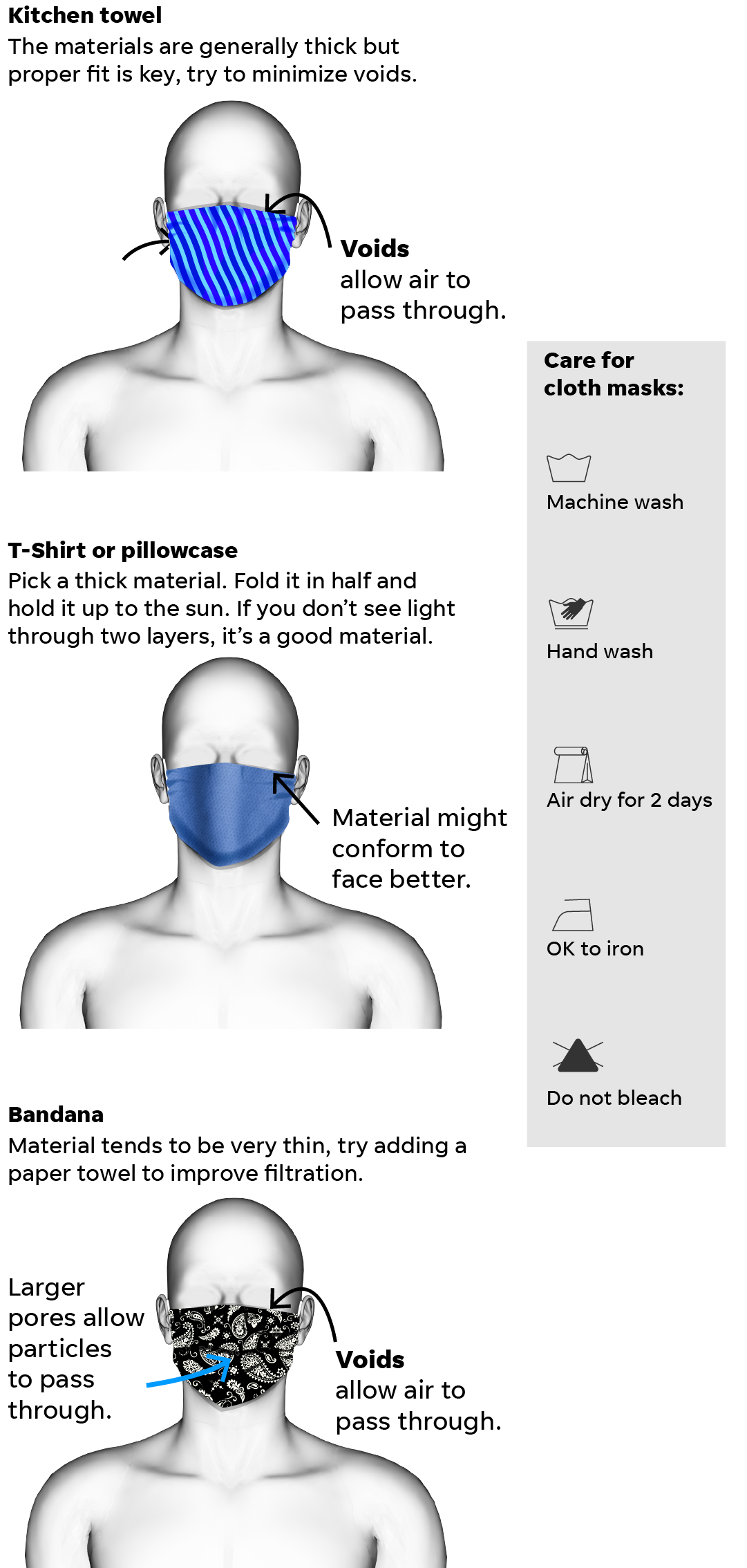
Coronavirus masks: How to clean, sanitize and reuse your mask
How to clean or sanitize a face mask
Hospitals Facing Coronavirus Are Running Out of Masks, Other Key Equipment - WSJ
Sanitizing Masks With Alcohol Degrades Performance – Smart Air
 How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling Stone
How to Wash Face Mask: Cleaning Tips, Disinfect Cloth Mask, Kill Germs - Rolling Stone



































Posting Komentar untuk "can i spray lysol on my mask"